The following is a modified van der Waals equation with an improved temperature dependence The usual van
Question:
The following is a modified van der Waals equation with an improved temperature dependence
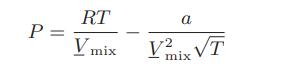
The usual van der Waals one-fluid mixing rules are used with this equation of state. Develop an expression for the mixture constant volume heat capacity of a mixure at elevated pressures in terms of the mixture volume Vmix, the mole fractions, temperature, the equation of state parameters, and each of the pure component ideal gas heat capacities.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:





