We have the following properties for a certain mixture for mixing at constant temperature and pressure: where
Question:
We have the following properties for a certain mixture for mixing at constant temperature and pressure:
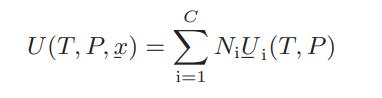
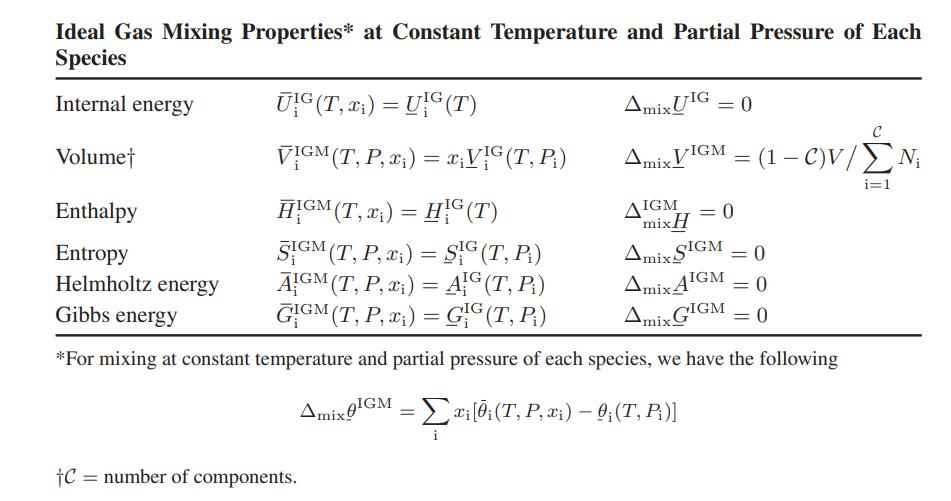
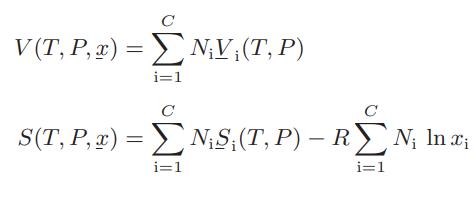
where Si, the pure-component molar entropy of component i, is given by
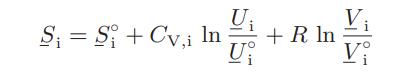 Here S°i , U°i , and V°i are the molar entropy, internal energy, and volume of pure component i in some reference state, and CV,i is its constant-volume heat capacity.
Here S°i , U°i , and V°i are the molar entropy, internal energy, and volume of pure component i in some reference state, and CV,i is its constant-volume heat capacity.
a. Obtain expressions for the partial molar volume, partial molar internal energy, partial molar entropy, and partial molar Gibbs energy of each component in this mixture in terms of S°i , U°i , V°i , CV,i, R, and T.
b. Obtain expressions for the volumetric and thermal equations of state for this mixture.
c. Obtain expressions for the enthalpy and the Helmholtz and Gibbs energies of this mixture.
Step by Step Answer:

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler





