The liquid in a two-phase, binary mixture of benzene and cyclohexane has a composition of 20 mol
Question:
The liquid in a two-phase, binary mixture of benzene and cyclohexane has a composition of 20 mol % of benzene and 80 mol % of cyclohexane at T = 80°C.
a. Find the pressure of the system and the composition of the vapor phase.
b. The vapor is removed, heated isobarically to 550 K, and passed through a reactor, where the following dehydrogenation reaction occurs:
![]()
If the system is in chemical equilibrium when leaving the reactor at 550 K, what is the composition of the species leaving the reactor?
c. The reactor stream is very quickly cooled (quenched) to 80°C so that the overall mixture composition remains the same as in part (b). Find the dew point pressure of this stream at 80°C, and the composition of the first drop of liquid that is formed.
Data:
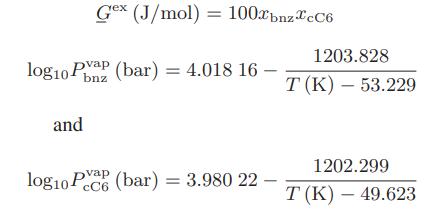
Step by Step Answer:

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler





