Use the data in problem 8.29 to compute the partial molar enthalpies of pyridine and acetic acid
Question:
Use the data in problem 8.29 to compute the partial molar enthalpies of pyridine and acetic acid in their mixtures at 25°C over the whole composition range.
Problem 8.29
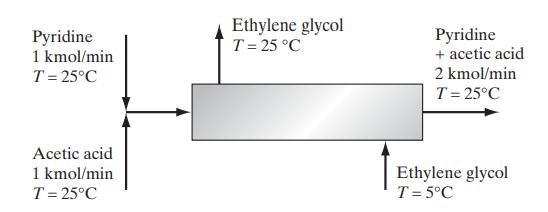
Two streams containing pyridine and acetic acid at 25°C are mixed and fed into a heat exchanger. Due to the heat-of-mixing effect, it is desired to reduce the temperature after mixing to 25°C using a stream of chilled ethylene glycol as indicated in the diagram. Calculate the mass flow rate of ethylene glycol needed. The heat capacity of ethylene glycol at these conditions is approximately 2.8 kJ/(kg K), and the enthalpy change of mixing (ΔmixH) is given below.
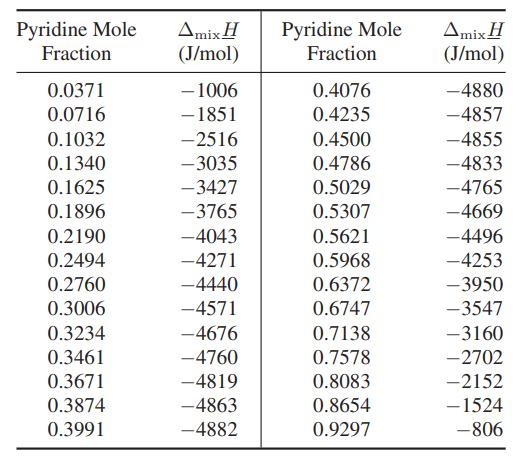
Data: Heat of mixing for pyridine (C5H5N) and acetic acid at 25°C [H. Kehlen, F. Herold and H.- J. Rademacher, Z. Phys. Chem. (Leipzig), 261, 809 (1980)].
Step by Step Answer:

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler





