A gas absorption tower is used to absorb sulfur dioxide from air into water at 20C and
Question:
A gas absorption tower is used to absorb sulfur dioxide from air into water at 20°C and 2.0 atm absolute pressure. At the top of the tower, the water is 100% pure and the gas contains 5.0 mol % SO2. At the bottom of the tower, the water contains 1.0 mol% SO2 while the gas contains 25 mol%
SO2. The individual mass transfer coefficients are = 5.4 mol/m2 s and
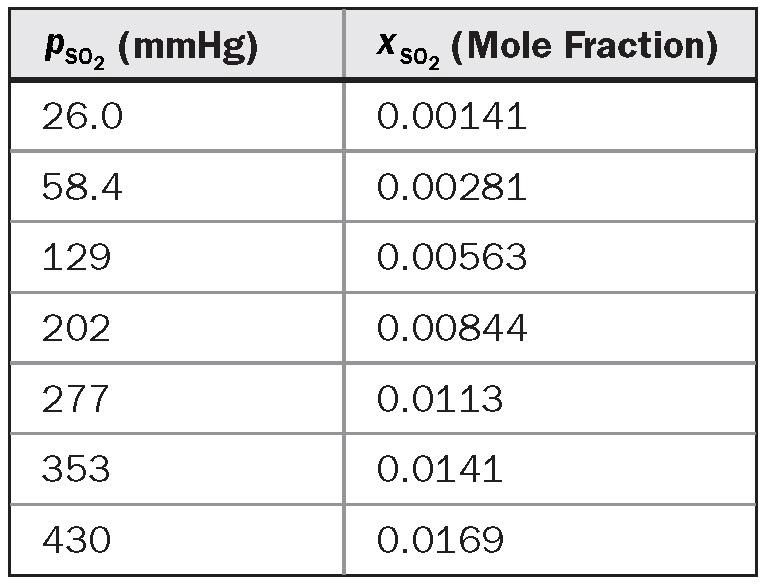
= 0.41 mol/m2 s for the liquid and the gas, respectively, and are constant throughout the tower. The equilibrium line is described by the data given in the following table. Find the overall mass transfer coefficients and and the interfacial composition at the top and at the bottom of the tower.
Equilibrium data for the system SO2 in water at 20°C are as follows:

Step by Step Answer:

Heat And Mass Transfer For Chemical Engineers Principles And Applications
ISBN: 9781264266678
1st Edition
Authors: Giorgio Carta





