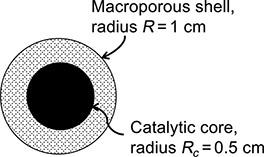
A superficially porous catalyst particle, sketched in Fig. P.11.12, consists of a solid spherical catalytic core surrounded
Question:
A “superficially porous” catalyst particle, sketched in Fig. P.11.12, consists of a solid spherical catalytic core surrounded by a spherical macroporous shell. The reaction 2 C2H4 → C4H8 occurs only on the surface catalytic core. The particle is placed in a rapidly flowing stream of pure C2H4 gas, which diffuses in the porous shell and reacts instantly as soon as it reaches the catalytic core. The absolute pressure is 2 atm, and the temperature is 400 K. The shell porosity is 0.5, and the tortuosity factor is 4. The system is isothermal. Assuming that the reaction is irreversible and that the overall process is diffusion-controlled:
(a) Write an equation to describe the steady-state flux of C2H4 in the shell for these conditions. (b)
Write a steady-state differential material balance for C2H4 along with boundary conditions needed to describe diffusional mass transfer in the shell.
(c) Integrate your material balance in part
(b) to determine the rate of mass transfer of C2H4.
(d) Calculate the rate of C4H8 production in mole/s.
FIGURE P.11.12:
Step by Step Answer:

Heat And Mass Transfer For Chemical Engineers Principles And Applications
ISBN: 9781264266678
1st Edition
Authors: Giorgio Carta





