Suppose that the data in Table 6.1 for the reaction of sulfuric acid with diethyl sulfate in
Question:
Suppose that the data in Table 6.1 for the reaction of sulfuric acid with diethyl sulfate in aqueous solution are described by a rate of the form r = kcAn. Investigate the consequences of an error of 1 percent in determining cA.
Table 6.1
Concentration of H2SO4 versus time for the reaction of sulfuric acid with diethyl sulfate in aqueous solution at 22.9◦C. Data of Hellin and Jungers, Bull. Soc. Chim. France, No. 2, pp. 386–400 (1957).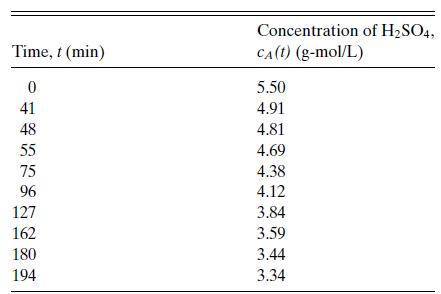
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





