Consider the full data set in Table 6.5 for the reversible reaction between sulfuric acid and diethyl
Question:
Consider the full data set in Table 6.5 for the reversible reaction between sulfuric acid and diethyl sulfate and test the applicability of each of the following rate expressions: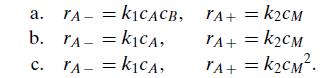
Table 6.5
Concentration of H2SO4 versus time for the reaction of sulfuric acid with diethyl sulfate in aqueous solution at 22.9◦C, full data
set. Data of Hellin and Jungers, Bull. Soc. Chim. France, No. 2, pp. 386–400 (1957).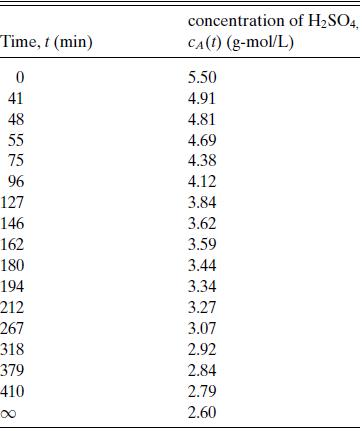
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





