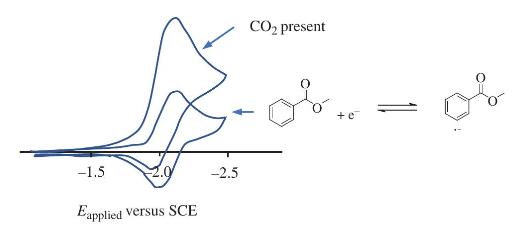
The cyclic voltammogram for methyl benzoate was recorded in dimethylformamide (DMF) using a platinum working electrode (lower
Question:
The cyclic voltammogram for methyl benzoate was recorded in dimethylformamide (DMF) using a platinum working electrode (lower curve). The product appears to be a singly-charged aryl radical. The solution was then exposed to \(\mathrm{CO}_{2}\) and a second voltammogram was scanned (upper curve).
Suggest a chemical explanation for the difference in behavior (in broad terms). Describe an experiment that would show support or refute your hypothesis.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Electroanalytical Chemistry Principles Best Practices And Case Studies
ISBN: 9781119538592,9781119538585
1st Edition
Authors: Gary A. Mabbott
Question Posted:





