The saponification of the ester propargyl acetate, with base (B, OH) was studied by Myers, Collett, and
Question:
The saponification of the ester propargyl acetate,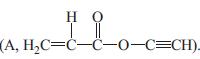
with base (B, OH–) was studied by Myers, Collett, and Lazzell using a conductivity technique to follow the course of the reaction. Conductivity, C(t), is related to the conversion of the base through the relation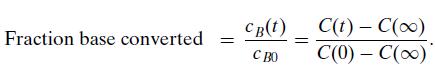
They report the data in Table 6.P5 for a solution that is initially 0.00873 N ester and 0.00673 N base. Find a rate expression that is consistent with these data.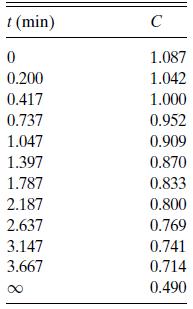
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





