In a process for the manufacture of acetone, acetone is separated from acetic acid by distillation. The
Question:
In a process for the manufacture of acetone, acetone is separated from acetic acid by distillation. The feed to the column is 60 mol percent acetone, the balance acetic acid.
The column is to recover 95% of the acetone in the feed with a purity of 99.5 mol percent acetone. The column will operate at a pressure of 760 mmHg, and the feed will be preheated to 70°C.
For this separation, determine
a. The minimum number of stages required;
b. The minimum reflux ratio;
c. The number of theoretical stages for a reflux ratio 1.5 times the minimum;
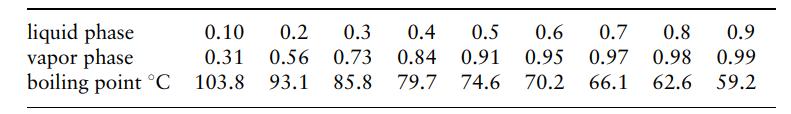
d. The number of actual stages if the plate efficiency can be taken as 60%. Equilibrium data for the system acetone-acetic acid, at 760 mmHg, mol fractions acetone:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





