Methyl ethyl ketone (MEK) is manufactured by the dehydrogenation of 2-butanol. A simplified description of the process
Question:
Methyl ethyl ketone (MEK) is manufactured by the dehydrogenation of 2-butanol. A simplified description of the process listing the various units used is given below:
1. A reactor in which the butanol is dehydrated to produce MEK and hydrogen, according to the reaction:
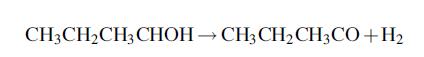
The conversion of alcohol is 88% and the selectivity to MEK can be taken as 100%.
2. A cooler-condenser, in which the reactor off-gases are cooled and most of the MEK and unreacted alcohol are condensed. Two exchangers are used but they can be modelled as one unit. Of the MEK entering the unit, 84% is condensed, together with 92% of the alcohol. The hydrogen is non-condensable. The condensate is fed forward to the final purification column.
3. An absorption column, in which the uncondensed MEK and alcohol are absorbed in water. Around 98% of the MEK and alcohol can be considered to be absorbed in this unit, giving a 10 wt% solution of MEK. The water feed to the absorber is recycled from the next unit, the extractor. The vent stream from the absorber, containing mainly hydrogen, is sent to a flare stack.
4. An extraction column, in which the MEK and alcohol in the solution from the absorber are extracted into trichloroethylane (TCE). The raffinate, water containing around 0.5 wt% MEK, is recycled to the absorption column.
The extract, which contains around 20 wt% MEK, and a small amount of butanol and water, is fed to a distillation column.
5. A distillation column, which separates the MEK and alcohol from the solvent TCE. The recovery of MEK is 99.99%. The solvent containing a trace of MEK and water is recycled to the extraction column.
6. A second distillation column, which produces a 99.9% pure MEK product from the crude product from the first column. The residue from this column, which contains the bulk of the unreacted 2-butanol, is recycled to the reactor.
For a production rate of 1250 kg/h MEK:
1. Draw a flow-sheet for the process.
2. Estimate the stream flow rates and compositions.
3. Estimate the reboiler and condenser duties of the two distillation columns.
4. Estimate the number of theoretical trays required in each column.
Step by Step Answer:

Chemical Engineering Design
ISBN: 9780081025994
6th Edition
Authors: Ray Sinnott, R.K. Sinnott, Sinnott Gavin Towler




