A good way to become familiar with thermodynamic processes is to start with a very simple system
Question:
A good way to become familiar with thermodynamic processes is to start with a very simple system and consider how various changes might affect it. Calculate the change in molar Gibbs free energy, ΔGm, for the process H2O(s) → H2O(l) at 1 atm and
(a) 10. °C;
(b) 0. °C. Decide for each temperature whether melting is spontaneous or not. Treat ΔHfus and ΔSfus as independent of temperature.
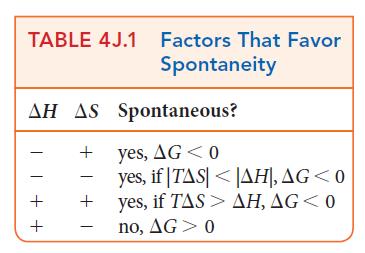
ANTICIPATE Because the solid and liquid phases are in equilibrium at the melting point, you should expect to find that ΔG = 0 at 0.°C. Above that temperature the melting of the solid state is favored, so you should expect that ΔG will be negative at 10.°C.
PLAN Find the enthalpy of fusion of water in Table 4C.1 (the values there are for 1 atm and the transition temperature). The entropy of fusion of water can be determined from the values in Table 4G.1. Use Eq. 3 to calculate the change in Gibbs free energy.![]()
Step by Step Answer:

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman





