Assume that H and S are independent of temperature and use data in Appendix 2A to calculate
Question:
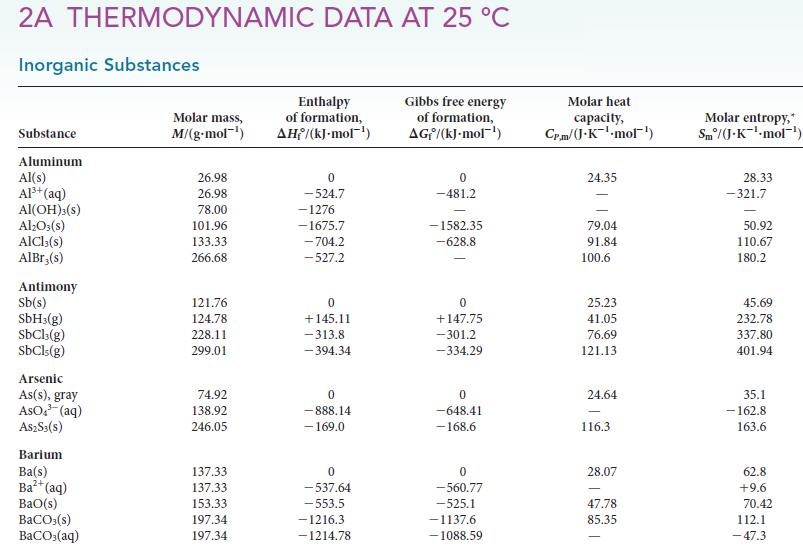
Assume that ΔH° and ΔS° are independent of temperature and use data in Appendix 2A to calculate ΔG° for each of the following reactions at 80.°C. Over what temperature range will each reaction be spontaneous under standard conditions?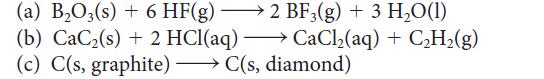
Transcribed Image Text:
(a) B₂O3(s) + 6 HF(g) → 2 BF3(g) + 3 H₂O(1) (b) CaC₂ (s) + 2 HCl(aq) →→→ CaCl₂(aq) + C₂H₂(g) diamond) - (c) C(s, graphite)C(s,
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (5 reviews)
a AG 9842 kJmol spontaneous ...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Assume that H and S are independent of temperature and use data in Appendix 2A to calculate G for each of the following reactions at 250. C. Over what temperature range will each reaction be...
-
Use the standard Gibbs free energies of formation in Appendix 2A to calculate G for each of the following reactions at 25 C. Comment on the spontaneity of each reaction under standard conditions at...
-
Use the standard Gibbs free energies of formation in Appendix 2A to calculate G for each of the following reactions at 25 C. Comment on the spontaneity of each reaction under standard conditions at...
-
Compute the least-squares regression line for predicting power (y) from wind speed (x).
-
Toyota Motor Corporation uses target costing. Assume that Toyota marketing personnel estimate that the competitive selling price for the Camry in the upcoming model year will need to be $22,000....
-
What is meant by the domain and range of a function?
-
It all started with a Facebook post. David Heath was scrolling Facebook one day when he read that socks are the number one item people in homeless shelters request. He remembers thinking that that...
-
Preparing and interpreting a statement of cash flows using a T-account work sheet Condensed financial statement data for Hale Company for the current year appear in it Exhibits 5.27 and 5.28. During...
-
Your 3 year old nephew got into the workbench and mixed iron filings into a container with sugar and marbles. Explain the steps you would take to separate and recover each substance (use point form...
-
Which of the following compounds become less stable with respect to their elements as the temperature is raised: (a) C 3 H 6 (g), cyclopropane; (b) CaO(s); (c) N 2 O(g); (d) HN 3 (g)?
-
Hydrochloric acid oxidizes zinc metal in a reaction that produces hydrogen gas and chloride ions. A piece of zinc metal of mass 8.5 g is dropped into an apparatus containing 800.0 mL of 0.500 m...
-
The American Red Cross is a supplier to the perfectly competitive domestic blood market. Unlike the other suppliers, however, the Red Cross is strictly nonprofitits goal is to sell as much blood as...
-
Suppose that the marginal distributions of v 1 and v 2 are standard normal distributions but that a Student's tcopula with four degrees of freedom and a correlation parameter of 0.5 is used to define...
-
Write the formulas for ex post computation of mean or average returns for security j for time t = 1, . . . , T periods and the sample variance of returns.
-
Assume that there are s states of nature. Write the formulas for the expected return of security j and the variance of security j returns. Next assume that there are two securities 1 and 2 in a...
-
The Maximo Business shows the following amounts in its owner's equity accounts at the end of December: R. Maximo, Capital, \(\$ 45080\); Revenues, \(\$ 80220\); Expenses, \(\$ 59920\). Required: Set...
-
A paragraph in the recent financial statements of Winfred Discount Stores begins: 'Advertising, selling, administrative and general expenses increased as a percentage of sales in 20X1 compared to...
-
Firm A makes and sells motorcycles. The total cost of each cycle is the sum of the costs of frames, assembly, and engine. The firm produces its own engines according to the cost equation: CE =...
-
On average there are four traffic accidents in a city during one hour of rush-hour traffic. Use the Poisson distribution to calculate the probability that in one such hour there arc (a) No accidents...
-
Consider a weak acid HA with a \(K_{a}\) value of \(1.6 \times 10^{-7}\). Calculate the \(\mathrm{pH}\) of a solution that is \(5.0 \times\) \(10^{-7} \mathrm{M} \mathrm{HA}\) and \(5.0 \times...
-
In Section 8.3 an equation was derived for the exact treatment of HA/NaA-type buffers. What would be the expression for \(\mathrm{B} / \mathrm{BHCl}\)-type buffers stated in terms of...
-
Which of the following mixtures would result in a buffered solution when \(1.0 \mathrm{~L}\) of each of the two solutions are mixed? a. \(0.1 \mathrm{M} \mathrm{KOH}\) and \(0.1 \mathrm{M}...
-
Tourists standing on a 100m tall viewing tower often drop coins into the fountain below. The height of a coin falling from the tower after t seconds is given by h(t) = 100 - 5t2. Find the...
-
The Association of National Advertisers found that media companies are using rebates to reward ad agencies for buying a certain amount of advertising time or space on behalf of their clients, the...
-
Imagine that you are the pilot of the light aircraft, which is capable of cruising at a steady speed of 300 kph in still air. There is enough fuel on board to last for four hours. Consider firstly...

Study smarter with the SolutionInn App


