Balance the following equations using the smallest whole number coefficients, then write the expression for K for
Question:
Balance the following equations using the smallest whole number coefficients, then write the expression for K for each reaction: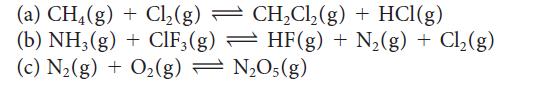
Transcribed Image Text:
(a) CH4(g) + Cl₂(g) — CH₂Cl₂(g) + HCl(g) (b) NH3(g) + ClF3(g) (c) N₂(g) + O₂(g)= HF (g) + N₂(g) + Cl₂(g) N₂O5(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
Lets balance the chemical equations and write the ...View the full answer

Answered By

Rinki Devi
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions.
Hi there! Are you looking for a committed, reliable, and enthusiastic tutor? Well, teaching and learning are more of a second nature to me, having been raised by parents who are both teachers. I have done plenty of studying and lots of learning on many exciting and challenging topics. All these experiences have influenced my decision to take on the teaching role in various capacities. As a tutor, I am looking forward to getting to understand your needs and helping you achieve your academic goals. I'm highly flexible and contactable. I am available to work on short notice since I only prefer to work with very small and select groups of students.
I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and helped them achieve great subject knowledge.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Balance the following equations using the smallest whole number coefficients, then write the expression for K for each reaction: (a) CH4(g) + O(g) (b) I(g) + F(g) IF, (g) (c) NO(g) + F(g) = FNO(g)...
-
1. Given Pascal's Triangle 4 1 3 1 2 6 1 3 1 5 10 10 4 1 5 1 1 a. Explain how the n and r values for C, relate to the number 6 shown above in Pascal's triangle. Justify your explanation by showing...
-
Balance the following equations using the method outlined in Section 3.7: (a) C + O2 CO (b) CO + O2 CO2 (c) C1 + Br2 HBr (d) K + C1O KOH + C1 (e) Mg + O2 MgO (f) O3 O2 (g) C1O2 C1O + O2 (h) N2 + C1...
-
In Problems 2628, find the value of each determinant. 21 5 0 26 -3 1 0
-
For the binary system ethanol(l)/isooctane(2) at 50?C, the infinite-dilution, liquid-phase activity coefficients are ?1? = 21.17 and ?2? = 9.84.(a) Calculate the constants A12 and All in the van Laar...
-
James Davis owns a small Internet service provider business. Recently, customers have been complaining that they are overcharged and are not receiving timely customer service. Billing rates seem to...
-
A mixture of \(\mathrm{H}_{2}\) and \(\mathrm{D}_{2}\) is contained in two bulbs connected by a porous plug. The bulbs are maintained at different temperatures of 200 and \(600 \mathrm{~K}\). The...
-
Consider the drug treatment system shown in the figure below. A hemispherical cluster of unhealthy cells is surrounded by a larger hemisphere of stagnant dead tissue (species B), which is turn...
-
3.On January 1, a company retired $800,000 face value bonds at a call price of 103. The bonds were originally issued for $848,000. On the retirement date the bonds had an unamortized premium of...
-
Complete the statement of cash flow for January through June on the CashFlow worksheet by completing the following tasks. As part of the process, you will need to use a circular reference. You will...
-
The normal boiling point of ethanol is 78.4 C. When 9.15 g of a soluble nonelectrolyte was dissolved in 100. g of ethanol, the vapor pressure of the solution at that temperature was 7.40 * 10 2 Torr....
-
A 0.124 m CCl 3 COOH(aq) solution has a freezing point of 20.423C. What is the percentage deprotonation of the acid?
-
Convert 729 10 to binary using both methods. Check your answer by converting back to decimal.
-
If 1 of the 839 challenges is randomly selected, find the probability of getting a challenge that was made by a male player or was rejected. Use the following results from the 839 player challenges...
-
Find the probability of being sentenced to prison, given that the subject entered a plea of guilty. Use the data in the accompanying table based on data from Does It Pay to Plead Guilty? Differential...
-
Find the probability of randomly selecting 1 of the 839 challenges and getting a challenge that was accepted, given that it was made by a female player. Use the following results from the 839 player...
-
Find the probability of being sentenced to prison, given that the subject entered a plea of not guilty. Use the data in the accompanying table based on data from Does It Pay to Plead Guilty?...
-
After comparing the results from Exercises 2 and 3, what do you conclude about the wisdom of entering a guilty plea? Use the data in the accompanying table based on data from Does It Pay to Plead...
-
Determine the present value in year 0 of the following cash flows using a periodic interest rate of 11% compoundedannually: YEAR 2 4 5 Receipts (S) Disbursements ($) 5,000 0 5,000 0 5,000 5,000 0...
-
A bubble-point liquid feed is to be distilled as shown in Figure. Use the Edmister group method to estimate the mole-fraction compositions of the distillate and bottoms. Assume initial overhead and...
-
Calculate the pressure exerted by benzene for a molar volume of 2.00 L at 595 K using the RedlichKwong equation of state: The RedlichKwong parameters a and b for benzene are 452.0 bar dm 6 mol 2 K...
-
The triphenylmethyl radical was the first radical to be observed. Draw all resonance structures of this radical, and explain why this radical is unusually stable:
-
Use the equation C P,m C V ,m = TV m 2 / and the Data Tables to determine C V ,m for H 2 O(l) at 298 K. Calculate (C p,m C V,m )/C P,m .
-
A dust storm destroys the majority of a farmer's peanut crop, causing him to raise the price of his peanut butter. What kind of shift does this create in the market for bread, a complement to peanut...
-
List the three or four basic "functions" of money - the roles it plays in the economy - and explain the meaning of each. Consider the interesting challenges that cryptocurrency offers to the...
-
Explain the Taylor Rule, and discuss the advantages and disadvantages of rules-based programs like this in contrast to allowing more discretion to Central Bank. officials.

Study smarter with the SolutionInn App


