Butadiene reacts to form its dimer according to the equation 2C 4 H 6 (g) C
Question:
Butadiene reacts to form its dimer according to the equation 2C4H6(g) → C8H12(g) The following data were collected for this reaction at a given temperature:
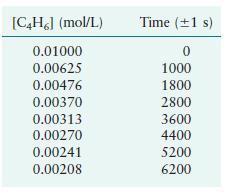
a. Is this reaction first order or second order?
b. What is the value of the rate constant for the reaction?
c. What is the half-life for the reaction under the conditions of this experiment?
Transcribed Image Text:
[C4H6] (mol/L) 0.01000 0.00625 0.00476 0.00370 0.00313 0.00270 0.00241 0.00208 Time (1 s) 0 1000 1800 2800 3600 4400 5200 6200
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
Figure 155 a To decide whether the rate law for this reaction is first o...View the full answer

Answered By

Vikash Gupta
I am graduated in Physics in 2018, from KIRORIMAL COLLEGE, University of Delhi. Now I am persuing Master's degree in physics. I like to do physics problems. I have experience of 1 year in tutoring. I think Physics is the only subject where you understand things,how they are happening . In physics you learn Maths and apply it. So I would like to join your platform to solve many Physics problems.
5.00+
5+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The tabulated data were collected for this reaction at a certain temperature: a. Determine the order of the reaction and the value of the rate constant at this temperature. b. What is the half-life...
-
The following data were collected for the rate of disappearance of NO in the reaction 2 NO(g) + O2(g) -- 2 NO2(g): (a) What is the rate law for the reaction? (b) What are the units of the rate...
-
The following data were collected in two studies of the reaction 2A + B C + D where In experiment 1, [B] 0 = 5.0 M. In experiment 2, [B] 0 = 10.0 M. a. Why is [B] much greater than [A]? b. Give the...
-
Consider the integral I = f(x) da where f(x) is the improper rational function (i) Use long division to rewrite f as the sum of a regular polynomial and a proper rational function. (ii) Factorise the...
-
Solve the equation y = xx2 + 1 / (ye y) and graph several members of the family of solutions (if your CAS does implicit plots). How does the solution curve change as the constant C varies?
-
Bells company had the following transactions in the month of May. May 2. Dee Bell transferred her personal car valued at $8,000 into the business. 10. Did $4,000 of work for a customer on account....
-
Use the data in Exercise 26 in Section 13.1 for the following. a. Compute a point estimate for the mean evaporation rate when the temperature is 20C. b. Construct a 99% confidence interval for the...
-
On February 25, Madison County Rocks Inc., a marble contractor, issued for cash 120,000 shares of $36 par common stock at $40, and on June 3, it issued for cash 50,000 shares of preferred stock, $8...
-
1.The Greenwood Company's balance sheet on June 30, 20X3, has total assets of $75,000, total liabilities of $30,000, paid-in-capital of $25,000, and retained earnings of $20,000. During the month of...
-
The balanced equation for the reaction of gaseous nitrogen dioxide and fluorine is The experimentally determined rate law is A suggested mechanism for this reaction is Is this an acceptable...
-
A certain first-order reaction has a half-life of 20.0 minutes. a. Calculate the rate constant for this reaction. b. How much time is required for this reaction to be 75% complete?
-
The balance sheet and income statement for the Papua New Guinea Coconut Company are as follows: Income Statement ($000) Balance Sheet (5000) Cash Accounts receivable Inventories $ 550 2,500 1.100...
-
Sandhill Truck Service uses the units-of-production method to calculate depreciation on its trucks. Each truck is expected to be driven 303,300 km over its life. Truck 10 was purchased on March 1,...
-
Establishment Industries borrows $1,040 million at an interest rate of 7.2%. It expects to maintain this debt level into the far future. Establishment will pay tax at an effective rate of 38%. What...
-
1. What type of case is this, a decision, Problem or scenario. 2. How should the RCB price themselves and what price and What type of pricing strategy should they go for: Value Based pricing,...
-
Official federal health care policy for the past 40 years has focused on market solutions, underlining the reliance on competition to improve quality and access and reduce costs. Has this economic...
-
When people are negotiating for themselvesfor example, buying a used mountain bicycle or exercise machinethey can determine the bargaining mix on their own. But when people negotiate in a...
-
Global Graphics Company was organized on January 1, 2002. At the end of the first 6 months of operations, the trial balance contained the following accounts. Debit Balance Total .......$109,100...
-
As of January 1, 2018, Room Designs, Inc. had a balance of $9,900 in Cash, $3,500 in Common Stock, and $6,400 in Retained Earnings. These were the only accounts with balances in the ledger on January...
-
An organic compound distilled from wood was found to have a molar mass of 32.04 g mol 1 and the following composition by mass: 37.5% C, 12.6% H, and 49.9% O. (a) Write the Lewis structure of the...
-
There are three isomers of dichlorobenzene, C 6 H 4 Cl 2 , which differ in the relative positions of the chlorine atoms on the benzene ring. (a) Which of the three forms are polar? (b) Which has the...
-
Predict which of the following pairs of ions would have the greatest coulombic attraction in a solid compound: (a) Mg 2+ , S 2+ ; (b) Mg 2+ , Se 2+ ; (c) Mg 2+ , O 2+ .
-
On 1/1/21, Vitale Co. invested $1,000,000 in Demo Co. for 25% of its outstanding stock. The equity method of accounting is appropriate. Demo Co. pays out 40% of net income in dividends each year....
-
2 E 1. Consider an 8PSK system. If the received symbols are s, (t) = cos(@t+ T 8 for i = 0,...,7. The constellation diagram is shown in the following figure. (15 points) 100 010 000 110 011 101 8PSK...
-
Happy Valley Henssupplies local restaurants and grocery stores with free-range eggs.Recently, they began raising baby chicks and hens for sale toconsumers at farmers markets. The market sellsbaby...

Study smarter with the SolutionInn App


