Calculate E cell for each of the following concentration cells: (a) Cu(s) Cu+ (aq, 0.0010 mol-L-)||Cu+ (aq,
Question:
Calculate Ecell for each of the following concentration cells: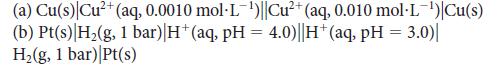
Transcribed Image Text:
(a) Cu(s) Cu²+ (aq, 0.0010 mol-L-¹)||Cu²+ (aq, 0.010 mol-L-¹')|Cu(s) (b) Pt(s) |H₂(g, 1 bar)|H*(aq, pH = 4.0)||H+ (aq, pH = 3.0)| H₂(g, 1 bar) Pt(s)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
a 0...View the full answer

Answered By

Benish Ahmad
I'm a professional software engineer. I'm lectutrer at GCUF and I have 3 years of teaching experience. I'm looking forward to getting mostly computer science work including:
Programming fundamentals
Object oriented programming
Data structures
object oriented design and analysis
Database system
Computer networks
Discrete mathematics
Web application
I am expert in different computer languages such as C++, java, JavaScript, Sql, CSS, Python and C#. I'm also have excellent knowledge of essay writing and research. I have worked in other Freelancing website such as Fiverr and Upwork. Now I have finally decided to join the SolutionInn platform to continue with my explicit work of helping dear clients and students to achieve their academic dreams. I deliver plagiarism free work and exceptional projects on time. I am capable of working under high pressure.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Looking at a simple box of name brand cereal, approxmately, what percentage of the retail price has gone towards the cos of advertising? O 90% O 30% 50% O 10%
-
Calculate the unknown concentration of the ion in each of the following cells: 2+ (a) Pb(s) Pb+ (aq, ?)||Pb+ (aq, 0.10 mol-L-) Pb(s), Ecell = +0.083 V. (b) Pt(s)| Fe+ (aq, 0.10 mol-L-), Fe+ (aq, 1.0...
-
PURPOSE This task helps you prepare for some group work in class. We opened the course by recognizing that we have gut reactions to business messages. Now that we've covered a good deal of theory...
-
Tubby Toys estimates that its new line of rubber ducks will generate sales of $7 million, operating costs of $4 million, and a depreciation expense of $1 million. If the tax rate is 35%, what is the...
-
Exercises in compound interest, no income taxes. To be sure that you understand how to use the tables in Appendix B at the end of this book, solve the following exercises. Ignore income tax...
-
Emarpy Appliance is a company that produces all kinds of major appliances. Bud Banis, the president of Emarpy, is concerned about the production policy for the companys best-selling refrigerator. The...
-
A sage of selling once said, Your job as a salesperson is to do 80 percent listening and 20 percent talking. Do you agree? Why or why not?
-
The device is used to hold an elevator door open. If the spring has stiffness k and it is compressed a distance , determine the horizontal and vertical components of reaction at the pin A and the...
-
Let W be the subspace spanned by 2 Find a basis for W and the dimension of W. (Note that the reduced row echelon form of (a) Basis: (b) Dimension: 0 3 6 0 0 Toon 3 [1-30 0 0 0 is 6 0 0 0 0 -1 1101...
-
Calculate the pH and pOH of (a) A solution that is 0.50 m NaHSO 4 (aq) and 0.25 m Na 2 SO 4 (aq); (b) A solution that is 0.50 m NaHSO 4 (aq) and 0.10 m Na 2 SO 4 (aq); (c) A solution that is 0.50 m...
-
Suppose that 1.436 g of impure sodium hydroxide is dissolved in 300. mL of aqueous solution and that 25.00 mL of this solution is titrated to the stoichiometric point with 34.20 mL of 0.0695 m...
-
In this exercise, you will create a program that allows the user to enter an employees gross pay amount as well as his or her filing status and number of withholding allowances. The program should...
-
The objective of this homework is to reinforce the scale space response extrema concept in key points detection, and ask you to compute a LoG pyramid for scale space extrema detection, and compare...
-
Feedback 1. The objective function can be expressed mathematically by multiplying the unit contribution margin by the units to be produced for each product and then summing over all products. An...
-
4. Study the equations for the two air pollution indices and answer the questions. Air Pollution Index (before 2013) 5 pollutants are included in the calculation of the Air Pollution Index (API)....
-
b) Fill in blank spaces in Table 1b and interpret the results. Given the following marketing report from a production firm, answer the following questions. Table 1a Confirmatory Specification with...
-
You will create a complete cash budget for the four quarters of 2022 for the above company. Assumptions used in the cash budgeting process include: 1) Actual and expected sales from the second...
-
You can never win by doing the right thing, is George Hamadas reaction when he received a summary of his units performance evaluation. George, who oversees a cost center, has a staff of 40, including...
-
Privitera and Freeman (2012) constructed a scale to measure or estimate the daily fat intake of participants; the scale was called the estimated daily intake scale for fat (EDIS-F). To validate the...
-
Draw all constitutional isomers with molecular formula C 3 H 9 N, and provide a name for each isomer.
-
Rank this group of compounds in order of increasing boiling point. -NH2 -N-
-
Identify whether each of the following compounds is expected to be water soluble: (a) (b) (c) -NH2 -NH2
-
A wire of circular cross-section carries current density that is not uniform but varies with distance from the center. this curren expressed as j(r) = A (1-7), where R is the radius of the wire and r...
-
Give a possible formula for the function shown in the figure below. 25 y 16 32 32 Round any calculated values to two decimal places. y = 25*5^b Q (40, 5) 48 Click if you would like to Show Work for...
-
Explain the relationship between compound interest and exponential growth.? provide Example

Study smarter with the SolutionInn App


