Calculate the standard enthalpy, entropy, and Gibbs free energy for each of the following reactions at 298
Question:
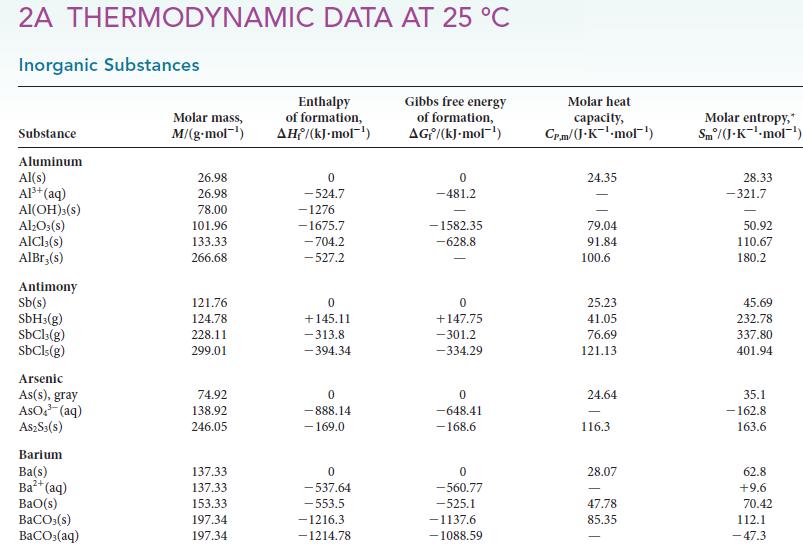
Calculate the standard enthalpy, entropy, and Gibbs free energy for each of the following reactions at 298 K by using data in Appendix 2A. For each case, confirm that the value obtained from the Gibbs free energies of formation is the same as that obtained by using the relation ΔG° = ΔH° – TΔS°. Which of the reagents in parts (a)–(c) would you expect to be the most effective at removing water from a substance? Explain your reasoning.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





