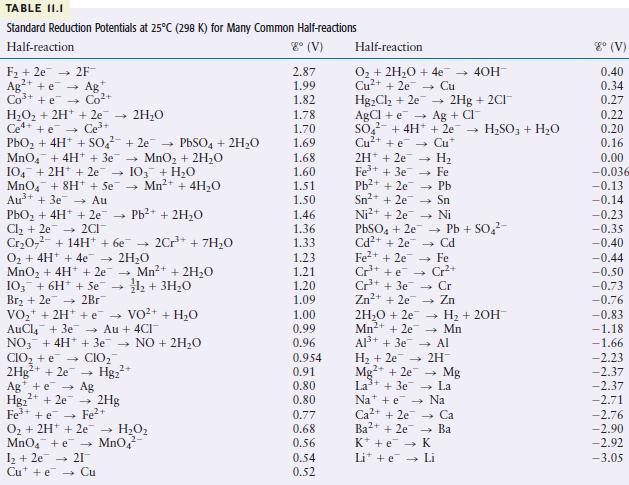
Consider only the species (at standard conditions) [mathrm{Na}^{+}, mathrm{Cl}^{-}, mathrm{Ag}^{+}, mathrm{Ag}, mathrm{Zn}^{2+}, mathrm{Zn} text {, and }
Question:
Consider only the species (at standard conditions)
\[\mathrm{Na}^{+}, \mathrm{Cl}^{-}, \mathrm{Ag}^{+}, \mathrm{Ag}, \mathrm{Zn}^{2+}, \mathrm{Zn} \text {, and } \mathrm{Pb}\]
in answering the following questions. Give reasons for your answers. (Use data from Table 11.1.)
a. Which is the strongest oxidizing agent?
b. Which is the strongest reducing agent?
c. Which species can be oxidized by \(\mathrm{SO}_{4}{ }^{2-}(a q)\) in acid?
d. Which species can be reduced by \(\mathrm{Al}(s)\) ?
Transcribed Image Text:
TABLE II.I Standard Reduction Potentials at 25C (298 K) for Many Common Half-reactions Half-reaction 8 (V) F +2e 2F Ag+ e Ag Co+ + e Co+ HO + 2H+ + 2e 2HO Ce4+ +e Ce+ PbO + 4H+ + SO +2e PbSO4 + 2HO MnO4 + 4H+ + 3e MnO + 2HO 104 + 2H+ + 2e 103 + HO MnO4 + 8H+ + Se Mn+ + 4HO Au+ + 3e Au PbO + 4H+ + 2e Pb+ + 2HO Cl +2e2CI CrO + 14H+ + 6e 2Cr+ + 7H0 O + 4H+ + 4e 2HO MnO + 4H+ + 2e 4 Mn+ + 2HO 1+ 3HO 103 + 6H + Se Br +2e 2Br VO + 2H+ + VO+ AuCl4 + 3e Au + 4CI NO3 + 4H+ + 3e NO + 2HO CIO + e CIO 2Hg+ + 2e Hg+ Ag + e 2+ Hg+ + 2e Fe+ + e O + 2H+ 2e MnO4 + e 1 +2e 21 Cute Cu Ag 2Hg Fe+ HO MnO4 + HO 2.87 1.99 1.82 1.78 1.70 1.69 1.68 1.60 1.51 1.50 1.46 1.36 1.33 1.23 1.21 1.20 1.09 1.00 0.99 0.96 0.954 0.91 0.80 0.80 0.77 0.68 0.56 0.54 0.52 Half-reaction O + 2HO + 4e Cu+ + 2e Cu HgCl +2e AgCl + e SO + 4H+ Cu+ + e 2H+2e7 4 H 1 Fe+ + 3e7 Pb+ + 2e7 Fe Pb - Sn Ni Sn+ + 2e Ni+ + 2e PbSO4 + 2e7 Cd+ + 2e Fe+ + 2e Cr+ + e Cr+ + 3e Zn+ + 2e7 2HO +2e Mn+ + 2e Al+ + 3e 2Hg + 2CI+ Ag + CI + 2e- HSO3 + HO Cu* Cd - Fe Cr+ Cr Zn - H + 2OH- Mn Al Pb + SO - 40H- H + 2e 2H Mg2+ + 2e La+ + 3e7 Nae Ca+ + 2e7 Ba2+ + 2e K + e Li + e Mg La Na Ca Ba K Li 8 (V) 0.40 0.34 0.27 0.22 0.20 0.16 0.00 -0.036 -0.13 -0.14 -0.23 -0.35 -0.40 -0.44 -0.50 -0.73 -0.76 -0.83 -1.18 -1.66 -2.23 -2.37 -2.37 -2.71 -2.76 -2.90 -2.92 -3.05
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
To answer these questions we will refer to the reduction potentials listed in the provided table Standard reduction potentials indicate the tendency o...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Consider the relation shown in Figure 30.2(d). How would it appear to a user with classification U? Suppose that a classification U user tries to update the salary of Smith to $50,000; what would be...
-
On October 10, the stockholders' equity section of Sherman Systems appears as follows. Common stock-$10 par value, 5,050 shares authorized, issued, and outstanding Paid-in capital in excess of par...
-
Consider only the species (at standard conditions) Br 2 , Br 2 , H + , H 2 , La 3+ , Ca, Cd in answering the following questions. Give reasons for your answers. a. Which is the strongest oxidizing...
-
Suppose there are two identical forest plots except that one will be harvested and left as is while the second will be cleared after the harvest and turned into a housing development. In terms of...
-
A gun fires a projectile of mass 10kg of the type to which the curves of Figure 2-3 apply. The muzzle velocity is 140m/s. Through what angle must the barrel be elevated to hit a target on the same...
-
When aniline is treated with fuming sulfuric acid, an electrophilic aromatic substitution reaction takes place at the meta position instead of the para position, despite the fact that the amino group...
-
Refer to the data for E5-16A. Flowever, instead of the FIFO method, assume Austins Jewelers uses the LIFO method. Requirements 1. Prepare a perpetual inventory record for the watches on the LIFO...
-
Suppose the following orders are received by an exchange for Cisco stock: Limit Order: Buy 200 shares at $25 Limit Order: Sell 200 shares at $26 Limit Order: Sell 100 shares at $25.50 Limit...
-
Summarize the key activities of any two or three agencies and commissions listed for the United States you identify as significant as well as one or two Canadian counterpart's and highlight...
-
Use the table of standard reduction potentials (Table 11.1) to pick a reagent that is capable of each of the following oxidations (under standard conditions in acidic solution). a. oxidizes...
-
The saturated calomel electrode, abbreviated SCE, is often used as a reference electrode in making electrochemical measurements. The SCE is composed of mercury in contact with a saturated solution of...
-
What are the advantages of using sample-and-hold operational transfer function (SHOTF) over the block pulse operational transfer function (BPOTF) in analyzing control systems involving...
-
You invested $8000 of your own money and borrowed $8000 from your broker to purchase shares of a company trading at a share price of $2. You paid 10% interest on your borrowed money for one year. You...
-
Write a MATLAB function/script that performs the following tasks. 2x Approximate: 24 dz. (a) Using the composite Trapezoidal rule with n = 8 (b) Using the composite Simpson's rule with n = 8 (c)...
-
What constitutes the central piece of guidance that a financial advisor can offer you? how can you party hard when you are in your 20s how can you save for your retirement how can you stop working...
-
What is Iris Inc. Total Assets? Accounts payable and accruals 65 Accounts receivable 54 Accumulated Depreciation(175) Cash 29 Common Stock 120 Fixed Assets (gross) 390 Inventory 128 Long-term Debt...
-
Determine how much needs to be invested if you want to end up with $5000 in interest after 7 years of simple interest at 1.8% APR. Starting investment= 39682.54 dollars. Your answer must be accurate...
-
Imagine that you are the head coach of a college sports team. One of your most important objectives is to win as many games as possible. Describe some controls that you would implement to help...
-
Repeat the previous problem, but close the positions on September 20. Use the spreadsheet to find the profits for the possible stock prices on September 20. Generate a graph and use it to identify...
-
Calculate the pH of 6.55 * 10 7 m HClO 4 (aq).
-
One of the largest uses of electricity is in the production of aluminum by electrolysis of its oxide dissolved in molten cryolite (Na 3 AlF 6 ). As an engineer, you might need to predict how much...
-
You are working in an analytical laboratory and have been asked to use the permanganate solution you prepared in Example 6K.1 to determine the concentration of bromide ions in a sample of groundwater...
-
Question 1.9 (3) The bar plot in code chunck 1-08 does not provide a quick, intuitive visualisation of the relationship between drive and number of cylinders. We want to understand whether there is a...
-
d The function, p(d)=1+ gives the pressure, in atmospheres (atm), at a depth d in the sea (d 33 is in feet). Note that p(0)=1 atm, p(33)=2, and so on. Find the pressure at 60 feet. The pressure at 60...
-
A compliance specialist must interpret laws and regulations to evaluate the impact of legal and regulatory requirements in their health care setting . Then, they explain the application to their...

Study smarter with the SolutionInn App


