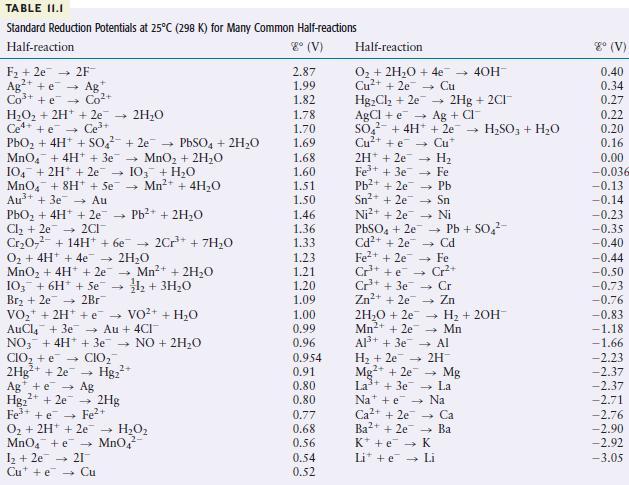
Use the table of standard reduction potentials (Table 11.1) to pick a reagent that is capable of
Question:
Use the table of standard reduction potentials (Table 11.1) to pick a reagent that is capable of each of the following oxidations (under standard conditions in acidic solution).
a. oxidizes \(\mathrm{Br}^{-}\)to \(\mathrm{Br}_{2}\) but does not oxidize \(\mathrm{Cl}^{-}\)to \(\mathrm{Cl}_{2}\)
b. oxidizes \(\mathrm{Mn}\) to \(\mathrm{Mn}^{2+}\) but does not oxidize \(\mathrm{Ni}\) to \(\mathrm{Ni}^{2+}\)
Transcribed Image Text:
TABLE II.I Standard Reduction Potentials at 25C (298 K) for Many Common Half-reactions Half-reaction 8 (V) F +2e 2F Ag+ e Ag Co+ + e Co+ HO + 2H+ + 2e 2HO Ce4+ +e Ce+ PbO + 4H+ + SO +2e PbSO4 + 2HO MnO4 + 4H+ + 3e MnO + 2HO 104 + 2H+ + 2e 103 + HO MnO4 + 8H+ + Se Mn+ + 4HO Au+ + 3e Au PbO + 4H+ + 2e Pb+ + 2HO Cl +2e2CI CrO + 14H+ + 6e 2Cr+ + 7H0 O + 4H+ + 4e 2HO MnO + 4H+ + 2e 4 Mn+ + 2HO 1+ 3HO 103 + 6H + Se Br +2e 2Br VO + 2H+ + VO+ AuCl4 + 3e Au + 4CI NO3 + 4H+ + 3e NO + 2HO CIO + e CIO 2Hg+ + 2e Hg+ Ag + e 2+ Hg+ + 2e Fe+ + e O + 2H+ 2e MnO4 + e 1 +2e 21 Cute Cu Ag 2Hg Fe+ HO MnO4 + HO 2.87 1.99 1.82 1.78 1.70 1.69 1.68 1.60 1.51 1.50 1.46 1.36 1.33 1.23 1.21 1.20 1.09 1.00 0.99 0.96 0.954 0.91 0.80 0.80 0.77 0.68 0.56 0.54 0.52 Half-reaction O + 2HO + 4e Cu+ + 2e Cu HgCl +2e AgCl + e SO + 4H+ Cu+ + e 2H+2e7 4 H 1 Fe+ + 3e7 Pb+ + 2e7 Fe Pb - Sn Ni Sn+ + 2e Ni+ + 2e PbSO4 + 2e7 Cd+ + 2e Fe+ + 2e Cr+ + e Cr+ + 3e Zn+ + 2e7 2HO +2e Mn+ + 2e Al+ + 3e 2Hg + 2CI+ Ag + CI + 2e- HSO3 + HO Cu* Cd - Fe Cr+ Cr Zn - H + 2OH- Mn Al Pb + SO - 40H- H + 2e 2H Mg2+ + 2e La+ + 3e7 Nae Ca+ + 2e7 Ba2+ + 2e K + e Li + e Mg La Na Ca Ba K Li 8 (V) 0.40 0.34 0.27 0.22 0.20 0.16 0.00 -0.036 -0.13 -0.14 -0.23 -0.35 -0.40 -0.44 -0.50 -0.73 -0.76 -0.83 -1.18 -1.66 -2.23 -2.37 -2.37 -2.71 -2.76 -2.90 -2.92 -3.05
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
We must search for reagents with reduction potentials larger than the reduction potentials of the s...View the full answer

Answered By

OTIENO OBADO
I have a vast experience in teaching, mentoring and tutoring. I handle student concerns diligently and my academic background is undeniably aesthetic
4.30+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Use the table of standard reduction potentials (Table 11.1) to pick a reagent that is capable of each of the following reductions (under standard conditions in acidic solution). a. reduces...
-
Use the table of standard reduction potentials (Appendix M) to calculate r G for the following reactions at 298 K. Data given in Appendix M (a) CIO3(aq) + 5 Cl(aq) + 6 H+ (aq) 3 Cl(g) + 3 HO(l) (b)...
-
Bromine is obtained from brine wells. The process involves treating water containing bromide ion with Cl 2 and extracting the Br 2 from the solution using an organic solvent. Write a balanced...
-
In Table 12. 1, when r = 0. 02, the present value of the cost rises for 68 years and then subsequently declines. Why? Table 12. 1 TABLE 12.1 Economic Harvesting Decision: Douglas Fir 10 20 30 40 50...
-
Show directly that the time rate of change of the angular momentum about the origin for a projectile fired from the origin constant g is equal to the moment force or torque about the origin.
-
Lidocaine is one of the most widely used local anesthetics. Draw the form of lidocaine that is expected to predominate at physiological pH. 'N. H. Lidocaine
-
Refer to the data for E5-16A. However, instead of the FIFO method, assume Austins Jewelers uses the average cost method. Requirements 1. Prepare a perpetual inventory record for the watches on the...
-
Consider the following two relations for Millennium College: Following is a typical query against these relations: a. On what attributes should indexes be defined to speed up this query? Give the...
-
What are the six gaps in the GAPS model. Suggest at least two ways to close each gap.
-
The amount of manganese in steel is determined by changing it to permanganate ion. The steel is first dissolved in nitric acid, producing \(\mathrm{Mn}^{2+}\) ions. These ions are then oxidized to...
-
Consider only the species (at standard conditions) \[\mathrm{Na}^{+}, \mathrm{Cl}^{-}, \mathrm{Ag}^{+}, \mathrm{Ag}, \mathrm{Zn}^{2+}, \mathrm{Zn} \text {, and } \mathrm{Pb}\] in answering the...
-
Explain the branch probabilities listed on this tree diagram, which models the outcomes of selecting two different students from a class of 7 juniors and 14 sophomores. 20 21 3 14 7 20 10 -or- 21 3...
-
A car suffers from 12% annual depreciation. If the initial value is $40,000, find the value after 4 years. Hint: Depreciation means inflation (losing money). Evaluate (2-)-. Simplify (2x). Show that...
-
Let G = (V, E) be an undirected graph. For any subset A, BCV, let C(A, B) denote the set of edges between A and B, i.e., C(A, B) = {e EE e connects some vertex in A and some vertex in B}. Note that,...
-
iversification at Ratcliffe Investments What's the current situation? Sports investors around the world have reaped unprecedented returns in recent years. In the National Basketball Association...
-
What percentage of your gross salary does the Consumer Financial Protection Bureau suggest your student loan payment be in order to be affordable and limit your risk of delinquency and default?
-
What marketing strategy and plan would you recommend for the new fitness tracking device startup company to generate awareness and drive sales with limited resources?
-
Perhaps your most important post-graduation objective is to get a job. Describe some control activities that you would pursue to help achieve this objective.
-
Use Stokes' Theorem to evaluate f(y+sin x) dx+(z+cos y) dy+rdz, where C is the rve r(t) = (sint, cost, sin 2t), t = [0, 2].
-
For the exercise in this topic, base your answers on the potentials listed in Table 6M.1 or Appendix 2B, with the exception of the reduction and oxidation of water at pH = 7: A 1.0 m KBr(aq) solution...
-
(a) What concentration (in moles per liter) of Ag + ions is required for the formation of a precipitate in 1.0 * 10 5 m NaCl(aq)? (b) What mass (in micrograms) of solid AgNO 3 needs to be added for...
-
Although molar solubilities are often of interest, you might find it difficult to locate the appropriate data. Solubility constants, however, are often easier to find, and can be converted to molar...
-
What steps are needed to find the minimized POS equation? BC A 00 01 11 10 00000 1 101 1
-
3. If f(x)=2x+1-5, determine f(8). x+4 4. State the restrictions on f(x)= 2x+8 5. Express f(x)=() as a power of 2. 6. Simplify, stating your answer using positive exponer 7. Simplify completely: 18
-
Use the Gauss-Jordan reduction to solve the following linear system: x1 3x1 -2x1 - x2 + 2x3 = - 27 4x2 + 8x3 = + 0x3 = -2

Study smarter with the SolutionInn App


