Consider the following mechanism for the hydrolysis of ethyl acetate. Write a complete balanced equation for the
Question:
Consider the following mechanism for the hydrolysis of ethyl acetate. Write a complete balanced equation for the overall reaction, list any intermediates, and identify the catalyst in this reaction.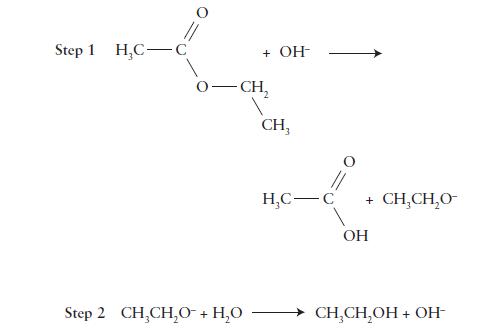
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





