Consider the question posed in Exercise 6N.19 except that a saturated calomel electrode (the solution is saturated
Question:
Consider the question posed in Exercise 6N.19 except that a saturated calomel electrode (the solution is saturated with KCl instead of having [Cl–] = 1.00 mol · L–1) is used in place of the standard calomel electrode. How will this replacement change your answers to Exercise 6N.19? The solubility of KCl is 35 g · (100 mL H2O)–1.
Exercise 6N.19
Suppose the reference electrode for Table 6M.1 were the standard calomel electrode, Hg2Cl2/Hg, Cl2([Cl2] = 1.00 mol · L–1), with its E° set equal to 0. Under this system, what would be the potential for
(a) The standard hydrogen electrode;
(b) The standard Cu2+/Cu redox couple?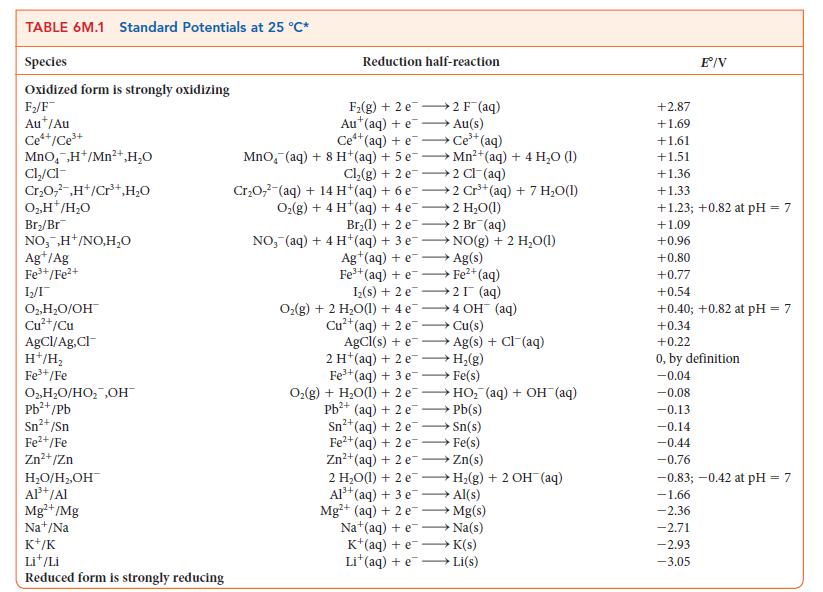
TABLE 6M.1 Standard Potentials at 25 °C* Species Oxidized form is strongly oxidizing F₂/F Aut/Au Ce*+/Ce³+ MnO,H+/Mn²+ H₂O Cl₂/cl- Cr₂O,²,H+/Cr³+,H₂O 0₂.H™/H₂O Br₂/Br NO,,H+/NO,H₂O Ag+/Ag Fe³+/Fe²+ 1₂2/1 O2, H₂O/OH Cu²+/Cu AgCl/Ag,Cl- H+/H₂ Fe³+/Fe O₂,H₂O/HO₂ ,OH™ Pb²+/Pb Sn²+ /Sn Fe²+/Fe Zn²+/Zn H₂O/H₂,OH™ Al³+ /Al Mg²+/Mg Na+/Na K+/K Lit/Li Reduced form is strongly reducing Reduction half-reaction F₂(g) +2 e Au (aq) + e 2 F¯ (aq) Au(s) Ce+ (aq) + e Ce³+ (aq) MnO, (aq) + 8 H+(aq) + 5 eMn²+(aq) + 4 H₂O (1) Cl₂(g) + 2 e 2 Cl¯(aq) Cr₂O7² (aq) + 14 H(aq) + 6 e2 Cr³+(aq) + 7 H₂O(1) O₂(g) + 4 H (aq) + 4e 2 H₂O(1) Br₂(1) + 2 e2 Br (aq) NO, (aq) + 4 H+(aq) + 3 e Ag+ (aq) + e NO(g) + 2 H₂O(1) Ag(s) Fe³+(aq) +e→→ Fe²+ (aq) Iz(s) + 2 e 2I (aq) O₂(g) + 2 H₂O(1) + 4e4 OH (aq) →→→ Cu(s) 2+ Cu²+(aq) + 2 e AgCl(s) + e 2 H (aq) + 2 e Fe³+(aq) + 3 e Ag(s) + Cl (aq) H₂(g) Fe(s) O₂(g) + H₂O(1) + 2 eHO₂ (aq) + OH¯(aq) Pb²+ (aq) + 2 e→→→→ Pb(s) Sn²+ (aq) + 2 e Sn(s) Fe²+ (aq) + 2 e →→→→Fe(s) Zn²+ (aq) + 2 eZn(s) 2 H₂O(1) + 2 eH₂(g) + 2 OH (aq) Al³+ (aq) + 3 e- →→→→Al(s) Mg²+ (aq) + 2 e→→→→→→ Mg(s) Na (aq) + e→→→→→→→ Na(s) K+ (aq) + eK(s) Lit(aq) +eLi(s) +2.87 +1.69 +1.61 +1.51 Eº/V +1.36 +1.33 +1.23; +0.82 at pH = 7 +1.09 +0.96 +0.80 +0.77 +0.54 +0.40; +0.82 at pH = 7 +0.34 +0.22 0, by definition -0.04 -0.08 -0.13 -0.14 -0.44 -0.76 -0.83; -0.42 at pH = 7 -1.66 -2.36 -2.71 -2.93 -3.05
Step by Step Answer:
In Exercise 6N19 the standard calomel electrode Hg2C12Hg C12C12 100 mol L 1 is used as the reference ...View the full answer



Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Students also viewed these Sciences questions
-
Internal alignment may be achieved by: O making cost of living adjustments. O matching competitors' wage rates. Opaying below-market base wages but offering training and rapid promotion. Omatching...
-
Suppose the reference electrode for Table 6M.1 were the standard calomel electrode, Hg 2 Cl 2 /Hg, Cl 2 ([Cl 2 ] = 1.00 mol L 1 ), with its E set equal to 0. Under this system, what would be the...
-
A chemist wishes to determine the concentration of CrO42- electrochemically. A cell is constructed consisting of a saturated calomel electrode (SCE; see Exercise 24) and a silver wire coated with...
-
The given graph is a transformation of one of the six basic functions. Find an equation for the given graph. 10 'y -1 -2 -3 -5 -6 -7 -8 -9 -10 -15 -13 -9 -8 -7 -6 -5 4 3 -2 -1 0 1 2 3 45 -11
-
A bat flying toward an obstacle at 12 m/s emits brief, high-frequency sound pulses at a repetition frequency of 80 Hz. What is the time interval between the echo pulses heard by the bat?
-
What is meant by constant opportunity costs and increasing opportunity costs? Under what conditions will a country experience constant or increasing costs?
-
Suppose that the current measurements in a strip of wire are assumed to follow a normal distribution with a mean of 10 milliamperes and a variance of 4 (milliamperes) \({ }^{2}\). What is the...
-
The 2006 AAPA survey of the population of physicians assistants who were working full time reported a mean annual income of $84,396 and standard deviation of $21,975. (Source: Data from 2006 AAPA...
-
Kohl's Department Store and Target Corporation buy their clothing from Form Fitters Inc., a merchandising firm, that has budgeted\ its activity for December according to the following information:\...
-
Tele Telcom, Inc. is a medium-sized company that produces automotive parts. The company has been in business for over 50 years and has a workforce of approximately 500 employees. Recently, some...
-
A sample of C 6 H 5 NH 3 Cl of mass 7.8 g is dissolved in water to make 350. mL of solution. What is the percentage deprotonation of the cations?
-
A sample of CH 3 NH 3 Cl of mass 15.5 g is dissolved in water to make 450. mL of solution. What is the pH of the solution?
-
Determine the two equations necessary to graph each hyperbola using a graphing calculator, and graph it in the viewing window indicated. y2 = 1; [-6.6, 6.6] by [-8, 8] 4 16 4+
-
Polaris Industries wishes to purchase a multiple-use in-plant road test simulator that can be used for ATVs, motorcycles, and snowmobiles. This is for research and experimentation and is considered...
-
A portable generator is needed at a remote construction site. Two alternatives are being considered. Their annual fixed costs and operating and maintenance costs per hour are shown in the table...
-
The Fence Company is setting up a new production line to create top rails. The relevant data for two alternatives are shown below. a. Based on MARR of 8 percent, determine the annual rate of...
-
Cowboy Metal Cutting produces a laser-cut part based on customer orders. The number of units requested on a customers order for the laser-cut part can vary from 1 to 150. Cowboy has determined that...
-
A utility is submitting their petition to their regulatory agency to justify rates for the upcoming year. Their proposal is based upon revenue requirements. The company has 45 percent of their...
-
Suppose that in past years the average price per square foot for warehouses in the United States has been $32.28. A national real estate investor wants to determine whether that figure has changed...
-
Problem 2. (0.6 points, 0.2 points for each question) (a) A company turns its inventory 2 times a month. Its months-of-supply = Its days-of-supply = Please show your analysis below: _months. days. (1...
-
For 1.25 mol of an ideal gas, P external = P =350. 10 3 Pa.The temperature is changed from 135C to 21.2C, and C V ,m = 3/2R. Calculate q, w, U, and H.
-
Suppose an adult is encased in a thermally insulating barrier so that all the heat evolved by metabolism of foodstuffs is retained by the body. What is her temperature increase after 2.5 hours?...
-
Draw bond-line structures for all constitutional isomers of C 5 H 12 ?
-
What ethical dilemmas arise in the context of organizational behavior, and how can ethical leadership models guide decision-making and promote ethical conduct within organizations ?
-
Sally earns gross wages of $1200 per week. She has standard deductions for social security and medicare. She is single with 1 allowance. She also has 6% of her gross wages put into a retirement...
-
Sunland Records wants to sell enough records to earn a profit of $139200. If the unit selling price is $22, unit variable cost is $10, and total fixed costs are $208800, how many records must Sunland...

Study smarter with the SolutionInn App


