For the exercise in this topic, base your answers on the potentials listed in Table 6M.1 or
Question:
For the exercise in this topic, base your answers on the potentials listed in Table 6M.1 or Appendix 2B, with the exception of the reduction and oxidation of water at pH = 7: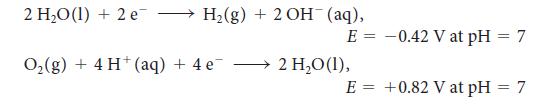 Copper from 200.0 mL of a solution of copper(II) sulfate is plated on to the cathode of an electrolytic cell.
Copper from 200.0 mL of a solution of copper(II) sulfate is plated on to the cathode of an electrolytic cell.
(a) Hydronium ions are generated at one of the electrodes. Is this electrode the anode or cathode?
(b) How many moles of H3O+ are generated if a current of 0.120 A is applied to the cell for 30.0 h?
(c) If the pH of the solution was initially 7.0, what will be the pH of the solution after the electrolysis? Assume no change in the volume of solution.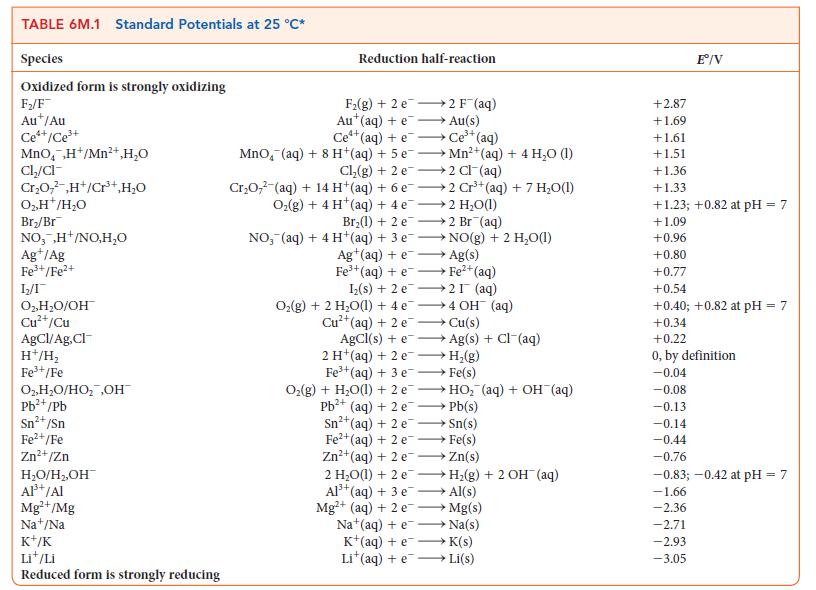
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





