Given the following data: calculate (Delta G^{circ}) for the reaction [6 mathrm{C}(s)+3 mathrm{H}_{2}(g) longrightarrow mathrm{C}_{6} mathrm{H}_{6}(l)] 2C6H6(l)
Question:
Given the following data:
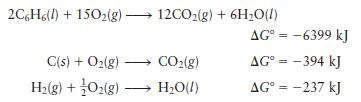
calculate \(\Delta G^{\circ}\) for the reaction
\[6 \mathrm{C}(s)+3 \mathrm{H}_{2}(g) \longrightarrow \mathrm{C}_{6} \mathrm{H}_{6}(l)\]
Transcribed Image Text:
2C6H6(l) +150(g) 12CO(g) + 6HO(l) C(s) + O(g) H(g) + O2(g) - CO(g) HO(l) AG = -6399 kJ AG = -394 kJ AG = -237 kJ
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
To calculate the standard Gibbs free energy change Delta Gcirc for the target reaction 6 textCs 3 textH2g ightarrow textC6textH6l We need to use the s...View the full answer

Answered By

Grace Igiamoh-Livingwater
I am a qualified statistics lecturer and researcher with an excellent interpersonal writing and communication skills. I have seven years tutoring and lecturing experience in statistics. I am an expert in the use of computer software tools and statistical packages like Microsoft Office Word, Advanced Excel, SQL, Power Point, SPSS, STATA and Epi-Info.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
You are a financial analyt in an investment advisory firm and you are helping your client to evaluate the value of a potential M&A target, WWG, inc. You estimate that WWG's enterprise value is $457.6...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-6. On December 12, Irene purchased the building where her store is located. She paid...
-
Suppose you come from a part of the world that is blessed with abundant water. Demand never comes close to the available amount. Should you be careful about the amount you use or should you simply...
-
A circuit with a nonlinear inductor can be modeled by the first-order differential equations. Chaotic oscillations for this situation have been extensively studied. Use a computer to construct the...
-
If the clinically desirable range for cholesterol is < 200 mg/dL, what proportion of people have clinically desirable levels of cholesterol? Cardiovascular Disease, Pulmonary Disease The duration of...
-
Darice Goodrich receives cash from customers as part of her job duties. Her other duty is to post the receipts to customer accounts receivable. Based on these duties, her company has weak a. ethics....
-
The financial statements of Marks and Spencer plc (M&S) are available at the books companion website or can be accessed at corporate.marksandspencer....
-
You are to interview a person who meets the following criteria. Has a career related to technology or industry (technology and industry as defined in the syllabus and R&D project instructions). Has...
-
For each of the following pairs, choose the substance with the higher positional probability (per mole) at a given temperature. a. solid CO 2 and gaseous CO 2 b. N 2 gas at 1 atm and N 2 gas at 1.0 ...
-
Calculate \(\Delta S_{\text {surr }}\) for the following reactions at \(25^{\circ} \mathrm{C}\) and \(1 \mathrm{~atm}\). a. C3H8(g) + 5O(g) 3CO2(g) + 4HO(l) b. 2NO2(g) 2NO(g) + O(g) AH = -2221 kJ AH...
-
Exercise 4.5 asks you to add the Exclusive state to the simple MSI snooping protocol. Discuss why this is much more difficult to do with the simple directory protocol. Give an example of the kinds of...
-
Expected return on security X is 2 0 % , expected return on security Y is 3 0 % , risk ( % ) of security X is 1 0 % and that of Y is 1 6 % . If coefficient of correlation between the returns of X and...
-
Assume you are the marketing manager for a new, high-end brand of jeans from the UK. A reason why selective distribution may be a better choice than exclusive distribution for a small unknown brand...
-
Your manager suggests determining the price based on a discounted dividend model and a discounted free cash flow valuation method. However, these two methods may produce very different estimates when...
-
A retaining wall .X m high was designed to stabilize a horizontal ground surface. The back of the wall is inclined 20 to vertical direction and can be assumed rough with interface friction angle of...
-
Joe Boylan purchased 600 common shares of Standard Cartons Inc. at the start of the year for $140 per share. He went on vacation in July and on his return was surprised to see that the shares were...
-
Divide into three groups. Each group must select one of the following types of CSR: ethical CSR, altruistic CSR or strategic CSR. Prepare a presentation arguing for the respective merits of each...
-
Write electron configurations for the following ions, and determine which have noble-gas configurations: (a) Cd2+ (b) p3- (c) Zr4+ (d) Ru3+ (e) As3- (f) Ag+
-
The following mechanism has been proposed for the formation of hydrazine in the overall reaction, N 2 (g) + 2 H 2 (g) N 2 H 4 (g): The rate law for the overall reaction is Rate = kr[N 2 ][H 2 ] 2 ....
-
The hydrolysis of sucrose (C 12 H 22 O 11 ) produces fructose and glucose: C 12 H 22 O 11 (aq) + H 2 O(l) C 6 H 12 O 6 (glucose, aq) + C 6 H 12 O 6 (fructose, aq). Two mechanisms are proposed for...
-
Some organic compounds containing the C=O group can react with themselves in a process known as aldol condensation. The mechanism for this reaction in acidic solution is shown here. Write the overall...
-
Once you have completed the Excel template, review the Excel data results and written analysis of the following ratios for 2018: Working Capital Current Ratio Quick Ratio Gross Profit % Net Profit %...
-
Using Microsoft Excel, prepare the following inventory control through the FIFO method and weighted average cost. The company Sina S.A DE C.V. began operations on February 28, 2019. In the month of...
-
Complete this form and upload into the assignment. of Account to Code Patient Acct # Disch Date A / R Days Total Charges Reason Code 2 3 5 9 6 0 0 XX / XX / XX 2 $ 2 3 5 , 6 5 4 . 3 3 2 , 1 2 3 5 9...

Study smarter with the SolutionInn App


