Indicators can be used to estimate the (mathrm{pH}) values of solutions. To determine the (mathrm{pH}) of a
Question:
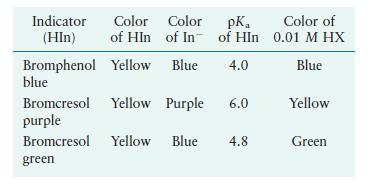
Indicators can be used to estimate the \(\mathrm{pH}\) values of solutions. To determine the \(\mathrm{pH}\) of a \(0.01 \mathrm{M}\) weak acid (HX) solution, a few drops of three different indicators are added to separate portions of \(0.01 \mathrm{M} \mathrm{HX}\). The resulting colors of the HX solution are summarized in the last column of the accompanying table. What is the approximate \(\mathrm{pH}\) of the \(0.01 \mathrm{M}\) HX solution? What is the approximate \(K_{\mathrm{a}}\) value for \(\mathrm{HX}\) ?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





