Methanol (CH 3 OH) is sometimes used as a fuel in high-performance engines. Using the data in
Question:
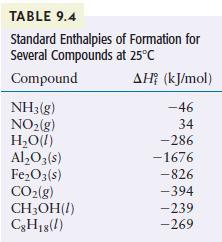
Methanol (CH3OH) is sometimes used as a fuel in high-performance engines. Using the data in Table 9.4, compare the standard enthalpy of combustion per gram of methanol with that of gasoline. Gasoline is actually a mixture of compounds, but assume for this problem that gasoline is pure liquid octane (C8H18).
Transcribed Image Text:
TABLE 9.4 Standard Enthalpies Several Compounds at 25C Compound NH3(g) NO(g) HO(1) AlO3(s) FeO3(s) CO(g) CHOH(1) C8H18(1) of Formation for AH (kJ/mol) -46 34 -286 -1676 -826 -394 -239 -269
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
The combustion reaction for methanol is 2CHOH1 30g 2COg 4HOl Using the standard enthalpies of format...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Methanol (CH3OH) has also been proposed as an alter-native fuel. Calculate the standard enthalpy of combustion per gram of liquid methanol, and compare this answer to that for ethanol in Exercise 80.
-
Methanol (CH3OH) is used as a fuel in race cars. (a) Write a balanced equation for the combustion of liquid methanol in air. (b) Calculate the standard enthalpy change for the reaction, assuming...
-
In January 1995, the Office of University Evaluation at Arizona State University surveyed faculty and staff members to find out their reaction to the closure of the university during Winter Break,...
-
Glass bottles can be either recycled (crushed and re-melted) or reused. The market will tend to choose the cheapest path. What factors will tend to affect the relative cost of these options? Is the...
-
Let the value of a in the logistic equation, Equation 4.46, be equal to 0.9, Make a map like that in Figure 4-21 when x1 = 0.4. Make the plot for three other values of x1 for which 0 < x1 < 1.
-
How did the decline in U.S. home prices in 20062008 affect aggregate demand?
-
Which method of estimating uncollectible receivables focuses on net credit sales? a. Aging approach b. Percent-of-sales approach C. Net realizable value approach d. All of the above
-
Review the chapter and make a list of all the advantages and disadvantages of matrix project organization you can find. Then add to the list any additional advantages or disadvantages that may have...
-
Implement the database in Oracle SQLPlus on arion.murdoch.edu.au (20 marks) All tables should be created as per your ERD; the marker will check your ERD against your tables. All integrity constraints...
-
Assuming that the combustion of hydrogen gas provides three times as much energy per gram as gasoline, calculate the volume of liquid H 2 (density = 0.0710 g/mL) required to furnish the energy...
-
Using the standard enthalpies of formation listed in Table 9.4, calculate the standard enthalpy change for the overall reaction that occurs when ammonia is burned in air to form nitrogen dioxide and...
-
Let d(t) be the number of dogs in the United States in year t, and let c(t) be the number of cats in the United States in year t, where t = 10 corresponds to 2010. (a) Find the function p(t) that...
-
A skyscraper has a mass of about 1.3010^8 kg and its center of mass is 147 m above the surrounding ground. How much gravitational potential energy (relative to the ground on which it is built) is...
-
ERP has been operating a large Platinum mine for many years. The company wants to acquire newly available equipment that will allow it to extract platinum ore from a currently inaccessible area of...
-
Reading material Raymond Frost, Alexa K. Fox & Judy Strauss (2019). Product: The Online Offer. E-Marketing , 9, 206 - 228. Raymond Frost, Alexa K. Fox & Judy Strauss (2019). Price: The Online Value....
-
Drawing on the session on sport marketing and the law, identify and discuss the various relevant legal issues in this scenario, including both tests and defenses, where applicable. What are the...
-
Compute the total depreciation allowances in respect of plant and machinery of Mr Cheung's business for the year of assessment 2010/11. Mr Cheung has not applied for application of s. 16G
-
Would the CSR policies of an organization influence your decision to use its products or services? Why or why not?
-
Suppose the concentration of glucose inside a cell is 0.1 mm and the cell is suspended in a glucose solution of 0.01 mm. a. What would be the free energy change involved in transporting 10-o mole of...
-
Write chemical equations for (a) The burning of lithium in oxygen; (b) The reaction of sodium metal with water; (c) The reaction of fluorine gas with water; (d) The oxidation of water at the anode of...
-
Identify the oxidation number of the halogen in (a) Hypoiodous acid; (b) ClO 2 ; (c) Dichlorine heptoxide; (d) NaIO 3 .
-
What are the sources for the production of helium and argon?
-
This case examines the October 2 0 1 5 initial public offering pricing decision for legendary Italian sports car company Ferrari by Fiat Chrysler management. We will evaluate Ferrari in light of...
-
You are thinking about buying BNS (Bank of Nova Scotia) stock. Their stock is currently trading for $81/share and pays and annual dividend of $4.12. You want to earn at least 8% on this investment in...
-
In order to discuss Zeffirelli's Hamlet as a film, you should answer the following questions: 1.Which scenes do you notice that are out of order? Which lines of dialogue are in the wrong scene? Note:...

Study smarter with the SolutionInn App


