Methanol is a high-octane fuel used in high-performance racing engines. Calculate G for the reaction given the
Question:
Methanol is a high-octane fuel used in high-performance racing engines. Calculate ΔG° for the reaction
![]()
given the following free energies of formation:
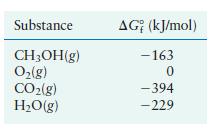
Transcribed Image Text:
2CHOH(g) + 30(g) 2CO(g) + 4HO(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
We use AG EAG products AG reactants 2AG COg 4AG H...View the full answer

Answered By

Muhammad Rehan
Enjoy testing and can find bugs easily and help improve the product quality.
4.70+
10+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The standard free energies of formation and the standard enthalpies of formation at 298 K for difluoroacetylene (C2F2) and hexafluorobenzene (C6F6) are For the following reaction: C6F6(g) 3C2F2(g) a....
-
The following data compare the standard enthalpies and free energies of formation of some crystalline ionic substances and aqueous solutions of the substances: (a) Write the formation reaction for...
-
In the Mond process for the purifi cation of nickel, carbon monoxide is reacted with heated nickel to produce Ni(CO)4, which is a gas and can therefore be separated from solid impurities: Ni(s) +...
-
Changing preferences can also affect changes in land use. In the United States, the proportion of the population in the 65-and-older age bracket is growing. What effects might this have on the...
-
Consider a projectile fired vertically in a constant gravitational field. For the same initial velocities, compare the times required for the projectile to reach its maximum height (a) For zero...
-
1. Prepare general journal entries for the following transactions of Valdez Services. a. The company paid $2,000 cash for payment on a 6-month-old account payable for office supplies. b. The company...
-
Motion Auto would like to assign the oldest costs of inventory items to its ending inventory. Which inventory costing method should Motion Auto choose?
-
Shim Company presents its statement of cash flows using the indirect method. The following accounts and corresponding balances were drawn from Shim's 2014 and 2013 year-end balance sheets: The income...
-
5. List 5 key external factors of your choice including both opportunities and threats you believe affect the firm and its industry. List the opportunities first and then the threats. 6. Explain your...
-
A chemical engineer wants to determine the feasibility of making ethanol (C 2 H 5 OH) by reacting water with ethylene (C 2 H 4 ) according to the equation Is this reaction spontaneous under standard...
-
Using the following data (at 25C),
-
Given a homogeneous system Ax = 0 of (scalar) linear equations, we say that a subset of these equations is irredundant provided that the corresponding column vectors of the transpose A T are linearly...
-
Suppose you purchased one share of ABC, Inc for $ 5 7 . 7 5 per share. The company paid a dividend of $ 3 . 2 9 per share during the year , and had an ending share price of $ 5 0 . 7 . What is the...
-
Take a look at how Dove is marketing the product, along with other men's products, in the videos found at: http://www.dovemencare.com/ ("Cleanse with Care", "The Right Shave," and "Moisturize Like a...
-
Gene has earnings of $550.00 this week. His year-to-date Employment Insurance premiums, for 2023, total $1,000.88. Calculate Genes Employment Insurance premium for the current pay period.
-
You borrow $550,000 at 3% APR to purchase a home. You plan to pay off the loan in 30 years with monthly payments of $ 2,310.00 Make an amortization table showing payments over the first three months....
-
In 2 0 2 1 , Black Ivy reported sales of $ 6 , 7 8 3 million, COGS $ 4 , 1 3 6 million, operating expense $ 1 , 6 9 9 million, interest expense $ 1 6 million. What is interest coverage ratio?
-
Why is managerial accounting relevant to business majors and their future careers?
-
Solve for the equilibria of the following discrete-time dynamical systems Pr pt+1 = Pr+2.0(I-Pr)
-
What is the molality of acetone, C 3 H 6 O, in an aqueous solution for which the mole fraction of acetone is 0.112?
-
Calculate the standard Gibbs free energy for each of the following reactions: 2 HI(g), K = 54 at 700. K (a) H(g) + 1(g) (b) CCl3COOH (aq) + HO(1) = CClCO (aq) + H3O+ (aq), K = 0.30 at 298 K
-
Permanganate ions are powerful oxidizing agents used in water treatment facilities to remove metals, such as iron, and toxic and malodorous chemicals, such as H 2 S. If you are using permanganate...
-
Quarry Ltd. is a mining company with several locations throughout Europe. Since its inception, they have always abided by the regulations of the various countries. With the introduction of...
-
Donald Sutton owns a commercial property that he acquired 10 years ago for $550,000 (the current UCC of the property is $300,000).The property was recently appraised and it was found that it has a...
-
Find the domain and range of the function given below: f(x) = 4x+1 The domain of f(x) is:

Study smarter with the SolutionInn App


