Using the following data (at 25C), Cdiamond (s) + O(g) Cgraphite + O(g) - '(s) CO(g) CO(g)
Question:
Using the following data (at 25°C),
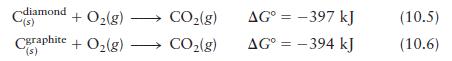
calculate ΔG° for the reaction
![]()
Transcribed Image Text:
Cdiamond "(s) + O(g) Cgraphite + O(g) - '(s) CO(g) CO(g) AG = -397 kJ AG = -394 kJ (10.5) (10.6)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
We reverse Equation 106 to make graphite a product as required and then ad...View the full answer

Answered By

Arun kumar
made more than four thousand assignments
5.00+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Vehicle air bags protect passengers by using a chemical reaction that generates gas rapidly. Such a reaction must be both spontaneous and explosively fast. A common reaction is the decomposition of...
-
Use data in Table 4H.1 or Appendix 2A to calculate the standard entropy change for each of the following reactions at 25C. For each reaction, interpret the sign and magnitude of the reaction entropy....
-
Use data in Table 4H.1 or Appendix 2A to calculate the standard reaction entropy for each of the following reactions at 25C. For each reaction, interpret the sign and magnitude of the reaction...
-
Suppose a state was trying to decide whether to fund primary and secondary education with a property tax or an income tax. What implications might this choice have for land use in the state?
-
A projectile is fired with a velocity v0 such that it passes through two points both a distance h above the horizontal. Show that if the gun is adjusted for maximum range, the separation of the...
-
A barometer indicates the atmospheric pressure to be 30.65 in of mercury. Calculate the atmospheric pressure in psia.
-
Tandrin Aviation Holdings Ltd. agreed to sell a jet aircraft to Aero Toy Store, LLC, for \($31.75\) million. ATS paid a \($3\) million deposit to a third party with the balance due upon delivery....
-
Sanyu Sony started a new business and completed these transactions during December. Dec. 1 Sanyu Sony transferred $65,000 cash from a personal savings account to a checking account in the name of...
-
Make relational model from this ER diagram 2. Convert the following ER diagram into relations/tables: row seat toCust SSNo Bookings Customers phone name Flights to Flt addr number aircraft day
-
Methanol is a high-octane fuel used in high-performance racing engines. Calculate G for the reaction given the following free energies of formation: 2CHOH(g) + 30(g) 2CO(g) + 4HO(g)
-
Consider the reaction carried out at 25C and 1 atm. Calculate H S, and G using the following data: 2SO2(g) + O2(g) 2SO3(g)
-
Find the perimeter and area of the composite figures 10 m 30 m 10 m
-
The name of our business is Boxology. Boxology is a recyclable packaging organization that contracts with big-name companies such as Amazon, FedEx, and UPS. Boxology will grow as these larger...
-
what should occur if a key piece of production equipment is repaired and recalibrated during a shift?
-
What is the government liable for if it terminates a contract for convenience? (HINT: They can't simply notify the contractor that they're terminating a contract and walk away.) Describe and analyze...
-
Creative Corner Inc. has $700,000 in earnings and excess cash of $500,000 and is trying to decide whether to pay out these funds to its shareholders in the form of dividends or reinvest it in the...
-
Write a Final Reflection Paper (approximately 3-5 pages). Your paper needs to include the following: What did you learn from reading this book? How will this book change how you work with students...
-
Oholics, Ltd., produces chocolate that it sells to candy makers. On April 1, it had no work-in-process inventory. It started production of 20,000 pounds of chocolate in April and completed production...
-
Solve for the equilibria of the following discrete-time dynamical systems Pr pt+1 = Pr+2.0(I-Pr)
-
Ethanol is produced for use as a fuel from corn and agricultural waste. Ethanol plant engineers need to know at what temperature ethanol boils at different pressures. The vapor pressure of ethanol at...
-
What is the vapor pressure of the solvent in each of the following solutions: (a) The mole fraction of glucose is 0.0316 in an aqueous solution at 40C; (b) An aqueous solution at 23C is 0.0240 m...
-
In a gas-phase equilibrium mixture of H 2 , Cl 2 , and HCl at 1000. K, [HCl] = 0.352 mmol L 1 and [Cl 2 ] = 7.21 mmol L 1 . Use the information in Table 5G.2 to calculate the equilibrium molar...
-
In the previous week's learning journal, we explored the initial steps of the accounting cycle. This week's journal serves as a continuation, where we will complete the accounting cycle for the...
-
2. Argon passes through a thermal expansion valve. Inlet conditions are 100 psi and 140F and the exit pressure is 15 psi. The diameter of the exit pipe is sufficiently larger than the inlet pipe so...
-
A standardized good is a good for which Blank______. Multiple choice question. any 2 units have the same features any 2 units have the same price all units are made at the same place all units are...

Study smarter with the SolutionInn App


