Refer to the illustration below for the reaction A D. (a) How many steps does this
Question:
Refer to the illustration below for the reaction A → D.
(a) How many steps does this reaction have?
(b) Which is the rate-determining step in this reaction?
(c) Which step is the fastest?
(d) How many intermediates must form in the reaction?
(e) A catalyst is added that accelerates the third step only. What effect, if any, will the catalyst have on the rate of the overall reaction?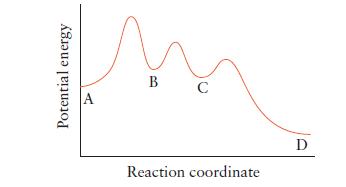
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





