The brilliant white glow of fireworks is due to the combustion of magnesium in air at a
Question:
The brilliant white glow of fireworks is due to the combustion of magnesium in air at a high temperature. But is that combustion spontaneous, in the thermodynamic sense, at ordinary temperatures? You can find out by calculating the total entropy change. Assess whether the combustion of magnesium, 2 Mg(s) + O2 (g) → 2 MgO(s), is spontaneous at 25°C under standard conditions, given ![]()
ANTICIPATE You can safely anticipate that all combustion reactions are spontaneous at normal temperatures.
PLAN Find the entropy change in the surroundings by using Eq. 2, then calculate the total entropy change by using Eq. 1.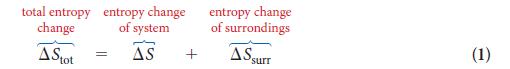
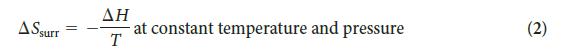
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





