The main buffer in the blood consists primarily of hydrogen carbonate ions (HCO 3 ) and
Question:
The main buffer in the blood consists primarily of hydrogen carbonate ions (HCO3–) and H3O+ ions in equilibrium with water and CO2: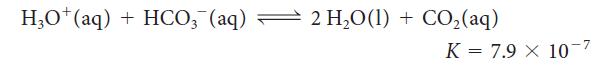
This reaction assumes that all H2CO3 produced decomposes completely to CO2 and H2O. Suppose that 1.0 L of blood is removed from the body and brought to pH = 6.1.
(a) If the concentration of HCO3 2 is 5.5 μmol · L–1, calculate the amount (in moles) of CO2 present in the solution at this pH.
(b) Calculate the change in pH that occurs when 0.65 mmol H3O+ is added to this sample of blood at this pH (that is, pH = 6.1). See Box 6G.1.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





