The overall reaction for the corrosion (rusting) of iron by oxygen is Using the following data, calculate
Question:
The overall reaction for the corrosion (rusting) of iron by oxygen is
![]()
Using the following data, calculate the equilibrium constant for this reaction at 25°C.
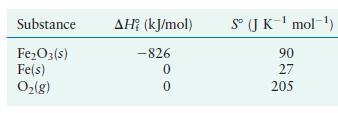
Transcribed Image Text:
4Fe(s) + 30(g) 2FeO3(s)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
We must first calculate ...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Find the value(s) of the variable. In Exercises 24-26, B and D are points of tangency. 14 7 C
-
You have fit a k-means algorithm with six clusters. The results are visualized below. Based on the plot, which statement is true? There is overlap between some clusters, which indicates there is no...
-
Synthesis Paper Write a three page synthesis paper that addresses the six trends and changes that Nagle cited. Use evidence from these four cases that explains the impact of these trends and changes...
-
Under Public Law 480, the United States sells surplus grains to developing countries, which pay in local currencies. Since the United States rarely spends all of these currencies, much of this grain...
-
A projectile is fired with initial speed v0 at an elevation angle of a up a hill of slope (a > ). (a) How far up the hill will the projectile land? (b) At what angle a will the range be a maximum?...
-
Draw the structure of the compound with molecular formula C8H11N that exhibits the following 1 H NMR and 13 C NMR spectra: Proton NMR 2 22 Chemical Shift (ppm) Carbon NMR 128.8 128.4 40.0 -126.1...
-
Mikes Powersports uses the (perpetual) LIFO inventory method. Mikes Powersports started August with 10 helmets that cost \($54\) each. On August 19, Mikes Powersports bought 15 helmets at \($56\)...
-
The following diagram shows a staged absorption column in which n-hexane (H) is absorbed from a gas into a heavy oil. A gas feed stream containing 5.0 mole% hexane vapor and the balance nitrogen...
-
In this specific case, what should be the key components of the physical distribution system? The situation: The ecological farmers association of Ontario is a non-for-profit organization that...
-
The value of K p is 3.7 10 6 at 900. K for the ammonia synthesis reaction. Assuming the value of H for this reaction is 92 kJ, calculate the value of K p at 550. K.
-
Consider the ammonia synthesis reaction where G =33.3 kJ per mole of N 2 consumed at 25C. For each of the following mixtures of reactants and products at 25C, predict the direction in which the...
-
A ray of light impinges from air onto a block of ice (n = 1.309) at a 60.0 angle of incidence. Assuming that this angle remains the same, find the difference 2, ice - 2, water in the angles of...
-
Last year, Wyani Company offered trade discounts of 15% and 12% on all its merchandise. This year the chain discount was changed to 13%, 12% and 5%. Is the amount of discount this year the same as...
-
Calculate the weighted-average cost of the following inventory purchases: Quantity Purchased Cost per Unit Total Amount June 41 30 $11.50 $345.00 June 14 40 $15.00 $600.00 June 29 30 $10.99 $329.70
-
A treasury note with a coupon rate of 16 1/4% that settles on February 15th, 2022 and matures on August 15, 2023. Assuming a quoted price on the note is 104.25, what is the yield on the note (using...
-
Consider the following packaging options for a beverage company. The selling prices, package sizes, and ecological impacts are provided in the table. Selling Price per package Package...
-
John Deere Inc is evaluating the revenues expected to be generated by a new product. They will evaluate it as if the product will generate $ 2 3 million per year in revenues for the first 1 0 years (...
-
Pick any large company and describe three risks that it faces and how it responds to those risks.
-
Determine whether the lines are parallel, perpendicular, or neither. 2x + 3y = -12, 2y - 3x = 8
-
A student was given a standard Cu(s)|Cu 2+ (aq) half-cell and another half-cell containing an unknown metal M in 1.00 m M(NO 3 ) 2 (aq) and formed the cell M(s)|M + (aq)||Cu 2+ (aq)|Cu(s). The cell...
-
You are working in the research laboratory of a company developing new forms of batteries for installation in satellites. As a part of your investigation, you have decided to study various...
-
The images below represent the solutes in the solutions of three acids (water molecules are not shown, hydrogen atoms and hydronium ions are represented by small gray spheres, conjugate bases by...
-
2. If a 3 by 3 matrix has det A = -1, find det(A), det(-A), det(A2), and det(A-1).
-
Prepare an analysis using quantitative (i.e., ROA, ROE, Profit Margin (please use financial data from annual reports)) and qualitative performance indicators to assess the company's 2022 performance...
-
Medication with strength 125 mg/5 mL has been ordered at 10 mg/kg. The patient weighs 101 lb. How much should be administered? (If less than 1, round to the nearest hundredth; otherwise, round to the...

Study smarter with the SolutionInn App


