The shapes of simple molecules are often relevant to their physical properties, and as you progress through
Question:
The shapes of simple molecules are often relevant to their physical properties, and as you progress through your study of chemistry you will see how important it is to know whether a molecule is planar or not. Predict the electron arrangement and the shape of a nitrogen trifluoride molecule, NF3.
ANTICIPATE The formula NF3 resembles that of ammonia, NH3, which is trigonal pyramidal; so you should suspect that NF3 is also trigonal pyramidal.
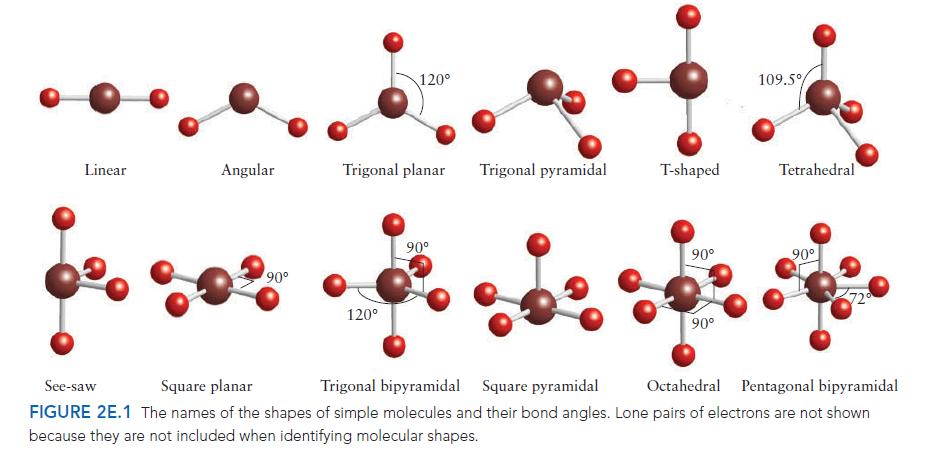
PLAN For the electron arrangement, draw the Lewis structure and then use the VSEPR model to decide how the bonding pairs and lone pairs are arranged around the central atom (consult Fig. 2E.2 if necessary). Identify the molecular shape from the layout of atoms, as in Fig. 2E.1.
FIGURE 2E.2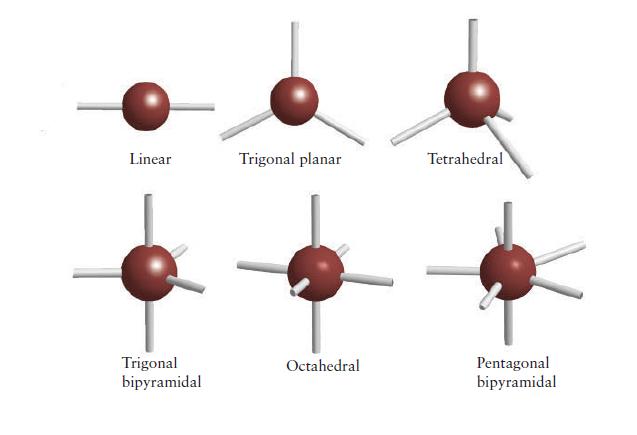
Step by Step Answer:

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman





