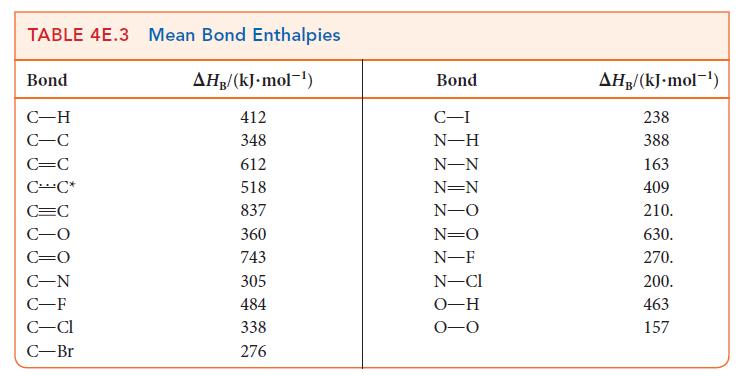
Use bond enthalpies (Tables 4E.2 and 4E.3) to estimate the reaction enthalpies for the hydrohalogenation of ethene
Question:
Use bond enthalpies (Tables 4E.2 and 4E.3) to estimate the reaction enthalpies for the hydrohalogenation of ethene by HX, where X = Cl, Br, I. What trend, if any, exists in these values?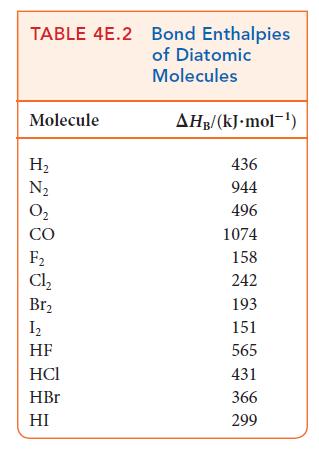
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





