Use the bond enthalpies in Tables 4E.2 and 4E.3 to estimate the reaction enthalpy for (a) 3
Question:
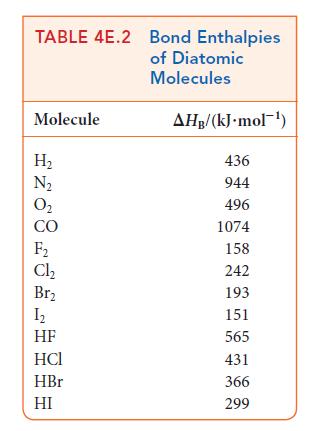
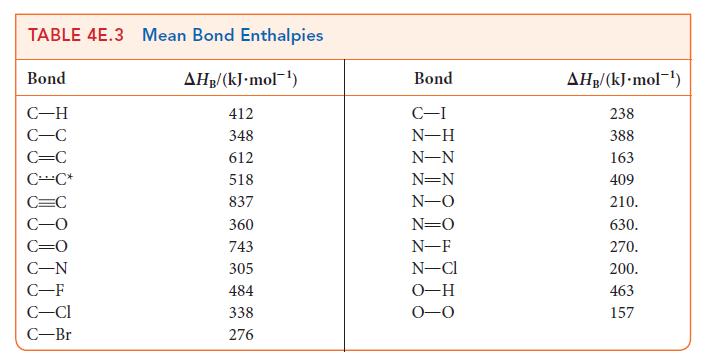
Use the bond enthalpies in Tables 4E.2 and 4E.3 to estimate the reaction enthalpy for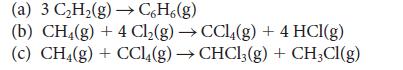
Transcribed Image Text:
(a) 3 C₂H₂(g) →CH(g) (b) CH4(g) + 4 Cl₂(g) → CCl4(g) + 4 HCl(g) (c) CH4(g) + CCl4(g) → CHCl3(g) + CH3CI(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
a 597 kJ ...View the full answer

Answered By

YOGENDRA NAILWAL
As I'm a Ph.D. student, so I'm more focussed on my chemistry laboratory. I have qualified two national level exams viz, GATE, and NET JRF (Rank 68). So I'm highly qualified in chemistry subject. Also, I have two years of teaching experience in this subject, which includes college teacher as well as a personal tutor. I can assure you if you hire me on this particular subject, you are never going to regret it.
Best Regards.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Mean bond enthalpies can be used to estimate the enthalpy of reaction when precise data are not available. Estimate the enthalpy of the reaction between liquid bromine and gaseous propene to form...
-
Use the data in Tables 4E.2 and 4E.3 to estimate the reaction enthalpy for (a) N(g) + 3 F(g) 2 NF3(g) (b) CH3CHCH(g) + HO(g) CH3CH(OH)CH3(g) (c) CH4 (g) + Cl(g) CHCl(g) + HCl(g)
-
The information in Table 4D.2 must be determined from experimental data, but because some reactions cannot be carried out directly, chemists who compile these types of tables commonly use enthalpies...
-
1. What is your brand/product? 2. Who is your target segment? 3. What are their needs/wants? 4. What message do you want to deliver to them?
-
The income statement for M2 Pizza Pie Company for the current year ended June 30 and balances of selected accounts at the beginning and the end of the year are as follows: Prepare the Cash Flows from...
-
Let f (x) = x 2 . Graph the functions f (x + 1), f (x - 1), f (x + 2), and f (x - 2). Make a guess about the relationship between the graph of a general function f (x) and the graph of f (g(x)),...
-
Find a recent sustainability report. Good reports can be found at the Australian Reporting Awards website; however, it might be useful to compare these to other firms reports. Required Prepare a...
-
Gruman Company purchased a machine for $ 220,000 on January 2, 2016. It made the following estimates: Service life 5 years or 10,000 hours Production 200,000 units Residual value $ 20,000 In 2016,...
-
How do you print the first value in a tuple called aVar?
-
Suppose that 50.0 g of water at 20.0 8C is mixed with 65.0 g of water at 50.0C at constant atmospheric pressure in a thermally insulated vessel. Calculate S and S tot for the process.
-
In 1750, Joseph Black performed an experiment that eventually led to the discovery of enthalpies of fusion. He placed two samples of water, each of mass 150. g, at 0.00 C (one ice and one liquid) in...
-
Discuss the provisions of Indian GAAP relating to classification of assets and liabilities. Give suitable examples.
-
Suppose that the opportunity-cost ratio for watches and cheese is 1C 1W in Switzerland but 1C 4W in Japan. At which of the following international exchange ratios (terms of trade) will Switzerland...
-
Recall the formula that states that $V = 1/P, where V is the value of the dollar and P is the price level. If the price level falls from 1 to 0.75, what will happen to the value of the dollar? a. It...
-
The current value of the Chemical Engineering Plant Cost Index (CEPCI) is 485. If at the same time next year the value has risen to 525 , what will be the average inflation rate for the year?
-
A friend of yours, Timorous ('Tim' for short) Ghostly, who has never taken an Accounting subject, has been assigned a short speech in his public speaking class. In this speech, Tim must describe the...
-
Suppose that the money supply is $1 trillion and money velocity is 4. Then the equation of exchange would predict nominal GDP to be: a. $1 trillion. b. $4 trillion. c. $5 trillion. d. $8 trillion.
-
Two firms dominate the market for surgical sutures and compete aggressively with respect to research and development. The following payoff table depicts the profit implications of their different R&D...
-
Outline a general process applicable to most control situations. Using this, explain how you would develop a system to control home delivery staff at a local pizza shop.
-
The reaction is the last step in the commercial production of sulfuric acid. The enthalpy change for this reaction is -227 kJ. In designing a sulfuric acid plant, is it necessary to provide for...
-
The bond energy for a C-H bond is about 413 kJ/mol in CH 4 but 380 kJ/mol in CHBr 3 . Although these values are relatively close in magnitude, they are different. Explain why they are different. Does...
-
An unknown compound contains only carbon, hydrogen, and oxygen. Combustion analysis of the compound gives mass percents of 31.57% C and 5.30% H. The molar mass is determined by measuring the...
-
An investor expects a stock to sell for $100 in exactly one year. The stock will not pay a dividend in the next year. After some research, the investor estimates the firm's beta as 1.20, the risk...
-
The decomposition of phosphorus pentachloride is described by PCl5(g) PC13(g) + Cl2(g) A sample of PCl5(g) at an initial concentration of 1.10 M is placed in a reaction vessel held at 250C. When...
-
Stone company produces electronic components. Lately, the company has been facing a drop in sales because of fierce competition and a downturn in the market. Consequently, Stone Corporation's cash...

Study smarter with the SolutionInn App


