Use the information in Table 5G.2 to determine the value of K at 300 K for the
Question:
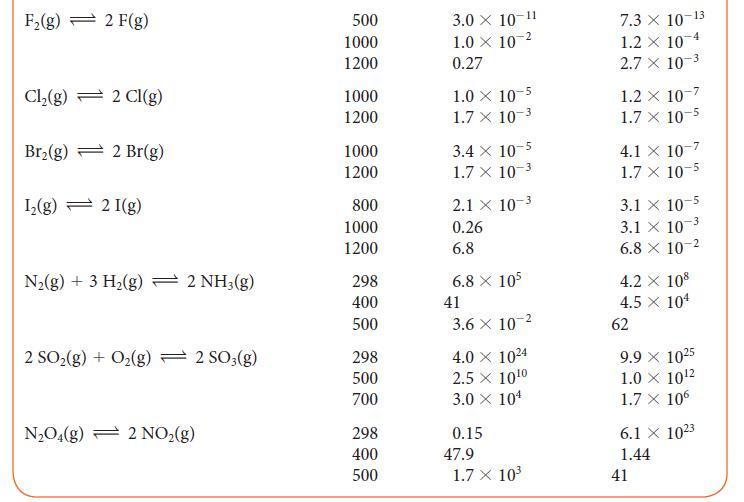
Use the information in Table 5G.2 to determine the value of K at 300 K for the reaction 2 BrCl (g) + H2(g) ⇌ Br2(g) + 2 HCl(g).
Transcribed Image Text:
TABLE 5G.2 Equilibrium Constants for Various Reactions Reaction H₂(g) + Cl₂(g) 2 HCl(g) H₂(g) + Br₂(g) → 2 HBr(g) H₂(g) + I₂(g) 2 HI(g) 2 BrCl(g) Br₂(g) + Cl₂(g) 2 HD(g) → H₂(g) + D₂(g) T/K* 300 500 1000 300 500 1000 298 500 700 300 500 1000 100 500 1000 K 4.0 × 10³1 4.0 × 10¹8 5.1 X 108 1.9 × 10¹7 1.3 × 10¹0 3.8 x 10¹ 794 160 54 377 32 5 0.52 0.28 0.26 K 4.0 × 10³1 4.0 × 10¹8 5.1 X 108 1.9 X 10¹7 1.3 X 10¹⁰ 3.8 x 10¹ 794 160 54 377 32 5 0.52 0.28 0.26
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
K ...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Use the information in Table 2.5 to predict the standard reaction enthalpy of2 H2 (g) + 02(g) 2 H2O (1) at 100C from its value at 25C.
-
(a) In an experiment, 5.0 mmol Cl 2 (g) was sealed into a reaction vessel of volume 2.0 L and heated to 1200. K, and the dissociation equilibrium was established. What is the equilibrium composition...
-
The reaction (CH3)3CBr + OH- (CH3)3COH + Br2 in a certain solvent is first order with respect to (CH3)3CBr and zero order with respect to OH-. In several experiments the rate constant k was...
-
The General Auditors Office (GAO) of ABC jurisdiction issued a report on the XYZ Electric Cooperative, a large member-owned utility. This report reviewed the work of MNO Consulting. MNO found...
-
(Multiple choice) (1) True or false: (a) The equivalent capacitance of two capacitors in parallel equals the sum of the individual capacitances. (b) The equivalent capacitance of two capacitors in...
-
1. Which arguments should be given more weight: those based on company policy, the employee handbook, and the labor agreement, or mitigating factors given by the grievant and his witnesses? Explain....
-
Consider a vapor condensing on a wall and forming a liquid film. Assume that locally the film thickness is related to the flow rate per unit transfer area by momentum-transfer considerations. Then...
-
a. Identify the main determinants for valuation of feature films, television programs, and general release feature productions by Columbia Pictures. b. Are the bases of valuation reasonable? Explain....
-
Rubbermaid has had an excellent track record in the development and launch of new products. Check out its most recent new product launches. What are the characteristics of its newest products?
-
The Ortega Food Company needs to ship 100 cases of hot tamales from its warehouse in San Diego to a distributor in New York City at minimum cost. The costs associated with shipping 100 cases between...
-
What is the molality of ethylene glycol, C 2 H 6 O 2 , in an aqueous solution used for antifreeze, given that the mole fraction of ethylene glycol is 0.250?
-
Use the vapor-pressure curve in Fig. 5A.3 to estimate the boiling point of water when the atmospheric pressure is (a) 60. kPa; (b) 160. kPa. FIGURE 5A.3 120 100 Vapor pressure, P/k Pa 40 20 101.325...
-
How many Btu of heat are required to change 9.00 lb of ice at 10F to steam at 232F?
-
The theta of a call option is -0.1. What does this mean?
-
Determine the impulse response of the following recursive system: \[y(n)-y(n-1)=x(n)-x(n-5) .\]
-
What is the delta of (a) a forward contract and (b) a futures contract on an asset that provides no income?
-
Determine the steady-state response of the system governed by the following difference equation: \[12 y(n)-7 y(n-1)+y(n-2)=\sin \left(\frac{\pi}{3} n ight) u(n) \text {. }\]
-
What is the relationship between theta, delta, and gamma when the underlying asset price follows geometric Brownian motion?
-
Your company worked on the projects shown as follows during the last year. Analyze the different profit centers based on their gross profit margins, their return on cash invested in the projects, and...
-
Listed below are common types of current liabilities, contingencies, and commitments: a. Accounts payable b. Bank loans and commercial paper c. Notes payable d. Dividends payable e. Sales and excise...
-
A van der Waals gas has a value of z = 1.00061 at 410. K and 1 bar and the Boyle temperature of the gas is 195 K. Because the density is low, you can calculate V m from the ideal gas law. Use this...
-
A sample of Na2SO4(s) is dissolved in 225 g of water at 298 K such that the solution is 0.325 molar in Na 2 SO 4 . A temperature rise of 0.146C is observed. The calorimeter constant is 330. J K 1 ....
-
Assign a name for each of the following compounds. a. b. c.
-
Determine the objective function for building a minimum-cost cylindrical tank of volume 160 ! and the height should be at least 2 longer than the radius, it should be noted that the cylindrical tank...
-
A pistoncylinder device initially contains 0 . 5 m 3 of nitrogen gas at 4 0 0 kPa and 2 7 C . An electric heater within the device is turned on and is allowed to pass a current of 2 A for 6 min from...
-
A polymer is injected into a mold at 205 C. The mold is 35 C. The part is 6 mm thick, and can be modeled as a plate. The glass transition temperature of the polymer is 125 C. The density of the...

Study smarter with the SolutionInn App


