Using the data in Table 11.1, calculate G for the reaction Is this reaction spontaneous? Cu+ (aq)
Question:
Using the data in Table 11.1, calculate ΔG° for the reaction
![]()
Is this reaction spontaneous?
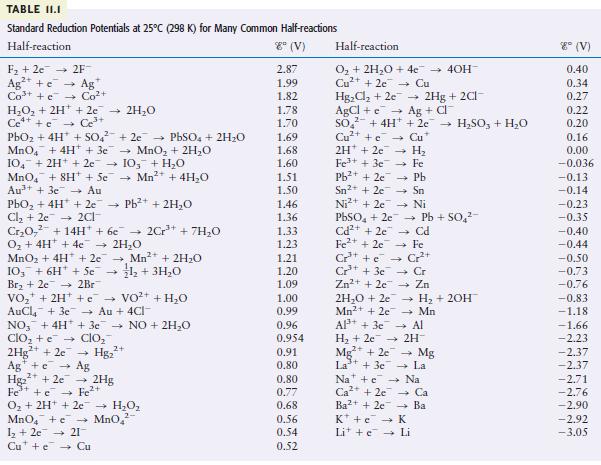
Transcribed Image Text:
Cu+ (aq) + Fe(s) Cu(s) + Fe+ (aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
The halfreactions are Cu 2e Cu Fe 2e Fe Cu Fe Cu Fe We can ...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Carbon disulfide (CS2) is a toxic, highly flammable substance. The following thermodynamic data are available for CS2(l) and CS2(g) at 298 K: (a) Draw the Lewis structure of the molecule. What do you...
-
Using the data in Table 116 on page 300, indicate the semiannual interest payment dates for the Motorola bonds that mature in 2031. (For the item in question, look under Interest Dates.) The two...
-
A 6 kg block of wood resting on a platform was hit by a 0.08 kg of bullet travelling upward with a speed of 951 m/s. The bullet emerges from the block with a speed of 783 m/s. How much force was...
-
Write a filter KamasutraCipher that takes two strings as command-line argument (the key strings), then reads strings (separated by whitespace) from standard input, substitutes for each letter as...
-
The speed of a particle of mass m varies with the distance x as v(x) = ax n. Assume v(x = 0) = 0 at t = 0. (a) Find the force F(x) responsible. (b) Determine x (t) and (c) F (t)
-
Explain why TCP, as a byte-oriented stream protocol, is not suitable for applications such as live or real-time multimedia streaming.
-
FRAUD PREVENTION AND DETERRENCE IN ACTION Assume the following facts: thirty-day collection period. The invoice is used to post-sales to the accounting system. Checks are received in the mailroom and...
-
A 10.0-m3 tank contains steam at 275C and 15.0bar. The tank and its contents are cooled until the pressure drops to 1.2bar. Some of the steam condenses in the process. (a) How much heat was...
-
Assume your health insurance has a $500 deductible and a 10% co-pay. How much would you have to pay for a $800 medical bill, assuming this is the only charge you have for the entire year?Disability...
-
Using the data from Table 11.1, predict whether 1 M HNO 3 will dissolve gold metal to form a 1 M Au 3+ solution. TABLE II.I Standard Reduction Potentials at 25C (298 K) for Many Common Half-reactions...
-
Describe completely the galvanic cell based on the following half-reactions under standard conditions: Ag + e Fe+ + e Ag Fe2+ 8 = 0.80 V 8 = 0.77 V (1) (2)
-
Consider the polynomials p 1 (t) = 1 + t 2 and p 2 (t) = 1- t 2 . Is {P 1 , P 2 } a linearly independent set in P 3 ? Why or why not?
-
What tradeoffs should be considered in evaluating the merits of adding additional CDCs? What costs are likely to increase and what costs are likely to decrease with this change? Beyond cost, what...
-
Many workplaces encourage their staff to build networks both internally and externally in the organization. This may be through online networks, such as LinkedIn, or by engaging with the community...
-
The following selected ledger accounts of Cameron Company are for February (the second month of its accounting year): Materials Inventory Feb. 1 balance 113,400 February credits 406,800 February...
-
Four sports teams offered Sam 5-year contracts to play football. All four offers include an upfront sign-up bonus, end of year annual payments, and end-of-the contract termination bonus. Sam wishes...
-
6) (10+20) The following theorem holds for any planar graph: There is a set of 0(n) vertices (called the separator set) whose removal from a n-vertex planar graph partitions the graph into disjoint...
-
When using past data to predict a cost that has fixed and variable components, it is possible to have an equation with a negative intercept. Does this mean that at a zero production level, the...
-
What is your opinion of advertising awards, such as the Cannes Lions, that are based solely on creativity? If you were a marketer looking for an agency, would you take these creative awards into...
-
Calculate the pH and pOH of (a) A solution that is 0.50 m NaHSO 4 (aq) and 0.25 m Na 2 SO 4 (aq); (b) A solution that is 0.50 m NaHSO 4 (aq) and 0.10 m Na 2 SO 4 (aq); (c) A solution that is 0.50 m...
-
Calculate E cell for each of the following concentration cells: (a) Cu(s) Cu+ (aq, 0.0010 mol-L-)||Cu+ (aq, 0.010 mol-L-')|Cu(s) (b) Pt(s) |H(g, 1 bar)|H*(aq, pH = 4.0)||H+ (aq, pH = 3.0)| H(g, 1...
-
Suppose that 1.436 g of impure sodium hydroxide is dissolved in 300. mL of aqueous solution and that 25.00 mL of this solution is titrated to the stoichiometric point with 34.20 mL of 0.0695 m...
-
Given: Truss with external loading as shown. Neglect the weight of the truss. Let F = 80 K. Find: The reactions at D and E. F 15A E * * 8ft" 8" 8ft"
-
Q5. (a) Natashah was studying the relationship between the price (RM y) of 1 kilogram of butter cake and the percentage of butter (x %) in the cake. Table 5.1 shows the data collected by Natashah for...
-
1. (10 points) A very large 250 mm-thick iron plate is cast by pouring iron into a sand mould at the temperature 40 C above the plate's melting temperature, so that the heat is withdrawn from both...

Study smarter with the SolutionInn App


