You continue exploring different combinations of electrodes in pursuit of better batteries. You know that the Ce
Question:
You continue exploring different combinations of electrodes in pursuit of better batteries. You know that the Ce4+/Ce3+ couple has been used in combination with Zn2+/Zn to develop new power sources with high potentials and a large storage capacity. Use the information in Appendix 2B to determine E8(Ce4+/Ce), for which the reduction half-reaction is![]()
PLAN Use the alphabetical listing in Appendix 2B to find half-reactions that can be combined to give the desired halfreaction. Combine these half-reactions and their standard Gibbs free energies of reaction. Use Eq. 1 of Topic 6L (ΔG° = –nFE°) to obtain the standard potentials and then simplify the resulting expressions. Because the constant F is used in the second and third steps (as set out in the text), it cancels, and you do not need to insert its numerical value.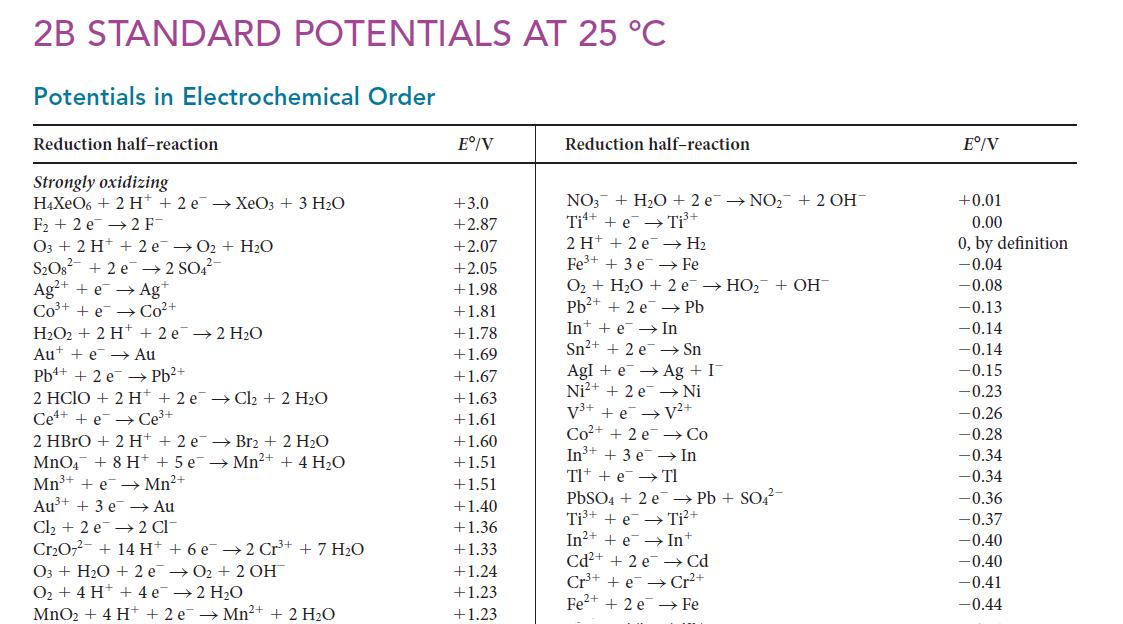
Step by Step Answer:

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman





