Find the overall order of the irreversible reaction from the following constant-volume data using equimolar amounts of
Question:
Find the overall order of the irreversible reaction
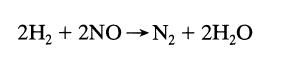 from the following constant-volume data using equimolar amounts of hydrogen and nitric oxide:
from the following constant-volume data using equimolar amounts of hydrogen and nitric oxide:
Transcribed Image Text:
2H₂ + 2NO N₂ + 2H₂O
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
To find the overall order of the reaction we can use the following equation rate kHnNOm where k is t...View the full answer

Answered By

Pushpinder Singh
Currently, I am PhD scholar with Indian Statistical problem, working in applied statistics and real life data problems. I have done several projects in Statistics especially Time Series data analysis, Regression Techniques.
I am Master in Statistics from Indian Institute of Technology, Kanpur.
I have been teaching students for various University entrance exams and passing grades in Graduation and Post-Graduation.I have expertise in solving problems in Statistics for more than 2 years now.I am a subject expert in Statistics with Assignmentpedia.com.
4.40+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
The following data where measured for the reaction BF3(g) + NH3(g) -- F3BNH3(g): (a) What is the rate law for the reaction? (b)What is the overall order of the reaction? (c) Calculate the rate...
-
1. Hydrogen peroxide reacts with thiosulfate ion in slightlyacidic solution as follows: H 2 O 2 + 2S 2 O 3 2? + 2 H + ? 2H 2 O + S 4 O 6 2? The reaction rate is independent of the hydrogen...
-
Can you say GDP is underestimated or overestimated using the Expenditures approach to calculating GDP? Why?
-
What does it mean that flame is non-luminous it is yellow it is invisible It has a very bright blue inner cone it is dark
-
A hedger takes a short position in five T-bill futures contracts at the price of 98 5/32. Each contract is for $100,000 principal. When the position is closed, the price is 95 12/32. What is the gain...
-
A 2.0-kg ball tied to a string fixed to the ceiling is pulled to one side by a force F. Just before the ball is released and allowed to swing back and forth, (a) How large is the force F that is...
-
For each of the following situations, calculate the \(t\)-statistic \((t)\) : a. \(\mathrm{X}^{-}=20.00 ; \mu=18 ; s \mathrm{X}^{-}=1.00\) b. \(X^{-}=20.00 ; \mu=13 ; s X^{-}=1.00\) c. \(X^{-}=12.00...
-
For each of the aids used in the case, describe how they were constructed and if there were any modifications in form. MINI CASE Heublein, Inc., develops, manufactures, and markets consumer food and...
-
What are the FASB codifications regarding auction rate securities, collateralized debt obligations, fixed-for-float OTC gas swap, and auction rate securities?
-
Aqueous A reacts to form R (A R) and in the first minute in a batch reactor its concentration drops from C A0 = 2.03 mol/liter to C Af = 1.97 mol/liter. Find the rate equation for the reaction if...
-
Snake-Eyes Magoo is a man of habit. For instance, his Friday evenings are all alike-into the joint with his week's salary of $180, steady gambling at "2-up" for two hours, then home to his family...
-
For the following exercises, determine whether the functions are one-to-one. f(x) = x 3
-
For the beam structure shown below, find all support reactions, draw the Shear Force Diagrams and the Bending Moment Diagrams (assuming El is constant for all members) using the following methods and...
-
The figure below represents a backfill behind a smooth vertical retaining wall. Estimate the magnitude and line of action of the lateral active force per meter length of the wall. What would be the...
-
The data in the following table were obtained in a travel time study on a section of highway with length 1.5 km, using the moving-vehicle technique. Determine average travel time, volume, and speed...
-
Water flows through the joint as shown below. Find the horizontal and vertical components of the force acting on the joint because of the flow of water. Neglect energy loss and body forces. A = 0.1 m...
-
B. Giulia runs at 4.5 m/s (1 m/s is 2.2 mi/h ... just think "about 2 mi/h"). She is running eastward into the sunrise and along a straight-line path. Show a complete sketch from when you start...
-
Calculate the NPV in Practice Problem 38 assuming a best case of the following: project life = 20 years; project beta = 0.8; SVn = $100,000; Rev1 = $500,000.
-
A Bloomberg Businessweek subscriber study asked, In the past 12 months, when traveling for business, what type of airline ticket did you purchase most often? A second question asked if the type of...
-
Describe how would your reactor volume and number of reactors change if you only needed 50% conversion to produce the 200 million pounds per year required?
-
If it takes 11 minutes to cook spaghetti in Ann Arbor, Michigan, and 14 minutes in Boulder, Colorado, how long would it take in Cuzco, Peru? Discuss ways to make the spaghetti more tasty. If you...
-
How do the steps in the design of a CSTR differ from those of a CSTR or a PFR with pressure drop?
-
You have sampled the soil of your garden and it has a soil organic carbon (SOC) content of 5.39 % SOC. Our class notes on organic matter give a simple method for converting between SOC and soil...
-
Mr. Ribaya would like to save Php. 500, 000 for his son's college education. How much should he deposit in a savings account every 6 months for 12 years if interest is at 1% compounded semi -...
-
A group of 16 people is choosing a chairperson and vice-chairperson. They put all 16 people's names into a hat. The first name drawn becomes chair. The second name drawn becomes vice-chair. How many...

Study smarter with the SolutionInn App


