Hydrogen sulfide is absorbed by a solution of methanolamine (MEA) in a packed column. At the top
Question:
Hydrogen sulfide is absorbed by a solution of methanolamine (MEA) in a packed column. At the top of the column, gas is at 20 atm and it contains 0.1% of H2S, while the absorbent contains 250 mol/m3 of free MEA. The diffusivity of MEA in solution is 0.64 times that of H2S. The reaction is normally regarded as irreversible and instantaneous.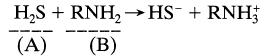
For the flow rates and packing used
(a) Find the rate of absorption of H,S in MEA solution.
(b) To find out whether it is worthwhile using MEA absorbent, determine how much faster is absorption with MEA compared to absorption in pure water. This problem was adapted from Danckwerts (1970).
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





