The gas-phase reversible reaction as discussed in Problem P11-7B A B is now carried out under
Question:
The gas-phase reversible reaction as discussed in Problem P11-7B A ⇄ B is now carried out under high pressure in a packed-bed reactor with pressure drop. The feed consists of both inerts I and species A with the ratio of inerts to the species A being 2 to 1. The entering molar flow rate of A is 5 mol/min at a temperature of 300 K and a concentration of 2 mol/dm3. Work this problem in terms of volume.
Additional information: (a) Co-current heat exchange
(a) Co-current heat exchange
(b) Countercurrent heat exchange.
(c) Constant heat-exchanger temperature Ta
(d) Compare and contrast each of the above results and the results for adiabatic operation (e.g., make a plot or a table of X and Xe obtained in each case).
(e) Vary some of the parameters, for example, (0 (f) Plot Qr and Ta as a function of V necessary to maintain isothermal operation.
Data from Problem P11-7B
The gas-phase reversible reaction A ⇄ B is carried out under high pressure in a packed-bed reactor with pressure drop. The feed consists of both inerts I and Species A with the ratio of inerts to the species A being 2 to 1. The entering molar flow rate of A is 5 mol/min at a temperature of 300 K and a concentration of 2 mol/dm3. Work this problem in terms of volume.
Additional information: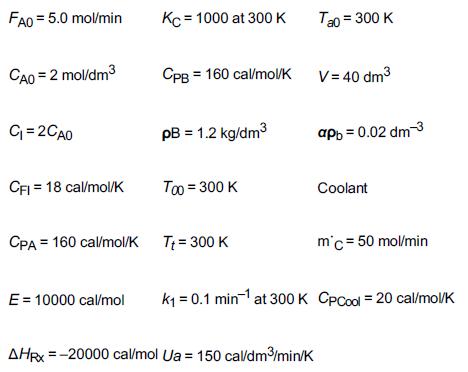
1. Adiabatic Operation. Plot X, Xe, p, T, and the rate of disappearance as a function of V up to V = 40 dm3. Explain why the curves look the way they do.
2. Vary the ratio of inerts to A (0≤ΘI≤10) and the entering temperature, and describe what you find.
3. Plot the heat that must be removed along the reactor (Q˙ vs. V) to maintain isothermal operation. Part (c) is “C” level of difficulty. We will continue this problem in Chapter 12.
Step by Step Answer:





