The elementary gas-phase reaction A + B 2C in Problem P11-8B is now continued and carried
Question:
The elementary gas-phase reaction A + B ⇄ 2C in Problem P11-8B is now continued and carried out in packed-bed reactor. The entering molar flow rates are FA0 = 5 mol/s, FB0 = 2FA0, and FI = 2FA0 with CA0 = 0.2 mol/dm3. The entering temperature is 325 K and a coolant fluid is available at 300 K.
Additional information: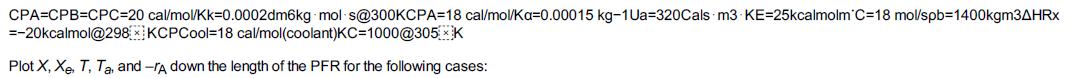
(a) Co-current heat exchange
(b) Countercurrent heat exchange
(c) Constant heat-exchanger temperature Ta
(d) Compare and contrast your results for (a), (b), and (c) along with those for adiabatic operation and write a paragraph describing what you find.
Data from Problem P11-8B
The elementary gas-phase reaction A + B ⇄ 2C is carried out in a packed-bed reactor. The entering molar flow rates are FA0 = 5 mol/s, FB0 = 2FA0, and FI = 2FA0 with CT0 =
a. Write the mole balance, the rate law, KC as a function of T, k as a function of T, and CA, CB, CC as a function of X, p, and T.
b. Write the rate law as a function of X, p, and T.
c. Show the equilibrium conversion is Xe=3KC4–(3KC4)2–2KC(FC4–1)2(KC4–1)
and then plot Xe versus T.
d. What are ΣΘiCPi, ΔCP, T0, entering temperature T1 (rate law), and T2 (equilibrium constant)?
e. Write the energy balance for adiabatic operation.
f. Case 1 Adiabatic Operation. Plot and then analyze Xe, X, p, and T versus W when the reaction is carried out adiabatically. Describe why the profiles look the way they do. Identify those terms that will be affected by inerts. Sketch what you think the profiles Xe, X, p, and T will look like before you run the Polymath program to plot the profiles
Additional information:
Step by Step Answer:





