The reaction A + B C + D is carried out adiabatically in a series of
Question:
The reaction A + B ⇄ C + D is carried out adiabatically in a series of staged packed-bed reactors with interstage cooling. The lowest temperature to which the reactant stream may be cooled is 27°C. The feed is equal molar in A and B, and the catalyst weight in each reactor is sufficient to achieve 99.9% of the equilibrium conversion. The feed enters at 27°C and the reaction is carried out adiabatically. If four reactors and three coolers are available, what conversion may be achieved?
Additional information: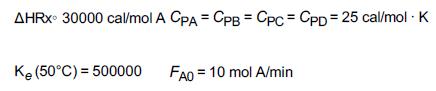
First prepare a plot of equilibrium conversion as a function of temperature.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





