Shows the temperatureconversion trajectory for a train of reactors with interstage heating. Now consider replacing the interstage
Question:
Shows the temperature–conversion trajectory for a train of reactors with interstage heating. Now consider replacing the interstage heating with injection of the feed stream in three equal portions, as shown in Figure P11-10A: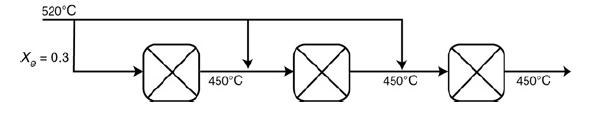
The figure shows an input feed of 520 degrees celsius supplied to three reactors. The three reactors are connected in series. A feed stream of 520 degrees celsius is supplied as the individual input of three reactors. The temperature of the output stream from each of these reactors is 450 degrees celsius. The adiabatic equilibrium conversion is 0.3. Sketch the temperature–conversion trajectories for
(a) An endothermic reaction with entering temperatures as shown,
(b) An exothermic reaction with the temperatures to and from the first reactor reversed, that is, T0 = 450°C.
Step by Step Answer:






