The reversible first-order gas reaction is to be carried out in a mixed flow reactor. For operations
Question:
The reversible first-order gas reaction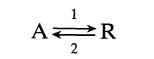
is to be carried out in a mixed flow reactor. For operations at 300 K the volume of reactor required is 100 liters for 60% conversion of A. What should be the volume of the reactor for the same feed rate and conversion but with operations at 400 K?![Data: k = 10 exp [-2416/T] ACp CPR CPA = 0 - AH, = -8000 cal/mol at 300 K K = 10 at 300 K Feed consists of](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1696/4/0/0/524651d048cabd501696400524239.jpg)
Transcribed Image Text:
1 A R 2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)

Answered By

Tobias sifuna
I am an individual who possesses a unique set of skills and qualities that make me well-suited for content and academic writing. I have a strong writing ability, allowing me to communicate ideas and arguments in a clear, concise, and effective manner. My writing is backed by extensive research skills, enabling me to gather information from credible sources to support my arguments. I also have critical thinking skills, which allow me to analyze information, draw informed conclusions, and present my arguments in a logical and convincing manner. Additionally, I have an eye for detail and the ability to carefully proofread my work, ensuring that it is free of errors and that all sources are properly cited. Time management skills are another key strength that allow me to meet deadlines and prioritize tasks effectively. Communication skills, including the ability to collaborate with others, including editors, peer reviewers, and subject matter experts, are also important qualities that I have. I am also adaptable, capable of writing on a variety of topics and adjusting my writing style and tone to meet the needs of different audiences and projects. Lastly, I am driven by a passion for writing, which continually drives me to improve my skills and produce high-quality work.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
On January 1, Innovative Solutions, Incorporated, issued $100,000 in bonds at face value. The bonds have a stated interest rate of 5 percent. The bonds mature in 10 years and pay interest once per...
-
300oC steam flows in a stainless-steel (ss.) pipe with thermal conductivity of 50 W/m.K. The pipe has internal diameter (ID) of 40 cm, and outer diameter (OD) of 42 cm. The pipe is placed in another...
-
Define Customer Evangelist? How can you create Customer Evangelist for your product or services? Explain it.
-
Use the graphs of f and g to solve Exercises 8390. y = g(x) HH y .y = f(x) # X
-
What purpose do protocols serve?
-
What fraction of vertical bars in Figure 4-5a is expected to include the population mean (10 000) if many experiments are carried out? Why are the 90% confidence interval bars longer than the 50%...
-
Suppose that two drugs A and B are to be tested on 12 subjects' eyes. The drugs will be randomly assigned to the left eye or right eye based on the flip of a fair coin. If the coin toss is heads then...
-
In Oregon, employers who are covered by the state workers' compensation law withhold employee contributions from the wages of covered employees at the rate of 2.8 for each hour or part of an hour...
-
A truck travels due east for a distance of 1.4 km, turns around and goes due west for 6.6 km, and finally turns around again and travels 2.7 km due east. (a) What is the total distance, in...
-
The first-order reactions are to be run in two mixed flow reactors in series anywhere between 10 and 90C. If the reactors may be kept at different temperatures, what should these temperatures be for...
-
On July 1, Paxson Corporation takes out a 12%, two-month, $50,000 loan at Friendly National Bank. Principal and interest are to be repaid on August 31. Required 1. Identify and analyze the...
-
The asset-liability approach for recording deferred income taxes is an integral part of generally accepted accounting principles. Instructions (a) Indicate whether each of the following independent...
-
The angular momentum of the propellers of a small airplane points directly forward from the plane. (a) In what direction do the propellers rotate as seen from the rear of the plane? (b) If the plane...
-
Standing on a round raft floating on a pond, how do you turn the raft around \(180^{\circ}\) ?
-
A motor drives a disk initially at rest through 23.9 rotations in \(5.0 \mathrm{~s}\). Assume the vector sum of the torques caused by the force exerted by the motor and the force of friction is...
-
A solid ball of inertia \(m\) rolls without slipping down a ramp that makes an angle \(\theta\) with the horizontal. (a) What frictional force is exerted on the ball? (b) As a function of \(\theta\),...
-
What maximum torque can a bicyclist deliver to the pedals?
-
What are the four phases of a forensic accounting engagement?
-
Differentiate. y = ln(3x + 1) ln(5x + 1)
-
If you need to determine the rate law, what methods would you use to collect the data and how would you analyze it?
-
Example 7-1. What is the error in assuming the concentration of species B is constant and what limits can you put on the calculated value of k? (i.e., k = 0.24 ?) Example 7-1 The liquid-phase...
-
1. Listen to the audios on the CRE Web site. Pick one and say why it could be eliminated. 2. Create an original problem based on Chapter 7 material. 3. Design an experiment for the undergraduate...
-
How does social constructionism elucidate the intricacies of knowledge production and the formation of subjective realities within diverse sociocultural contexts?
-
Years Estimated Useful Life of Assets 20 16 12 22 4 $200,000 $175,000 $150,000 $125,000 $100,000 $75,000 $50,000 $25,000 Purchase Price & Estimated Salvage Value Building Equipment Truck 0 Purchase...
-
Wilmington Company has two manufacturing departments-Assembly and Fabrication. All of its manufacturing overhead costs are fixed costs. The first set of data shown below is based on estimates from...

Study smarter with the SolutionInn App


