Write the rate law for the following reactions assuming each reaction follows an elementary rate law. Give
Question:
Write the rate law for the following reactions assuming each reaction follows an elementary rate law. Give the units of kA for each, keeping in mind some are homogeneous and some reactants are heterogeneous.
a. C2H6 → C2H4 + H2
b.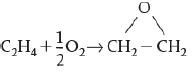
c. (CH3)3COOC (CH3)3 ⇄ C2H6 + 2CH3COCH3
d. nC4H10 ⇄ iC4H10
e. CH3COOC2H5 + C4H9OH ⇄ CH3COOC4H9 + C2H5OH
f. 2CH3NH2⇄cat(CH3)2NH+NH3
g. (CH3CO)2O + H2O ⇄ 2CH3COOH
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





