A 0.229-g sample of an unknown monoprotic acid is titrated with 0.112 M NaOH. The resulting titration
Question:
A 0.229-g sample of an unknown monoprotic acid is titrated with 0.112 M NaOH. The resulting titration curve is shown here.
Determine the molar mass and pKa of the acid.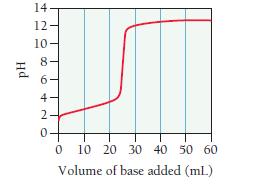
Transcribed Image Text:
Hd 14 12- 10 8 6- Na 00 4- 0 0 10 20 30 40 50 60 Volume of base added (ml)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
pK...View the full answer

Answered By

Ankit Mahajan
I am an electrical engineering graduate from Thapar institute of engineering and technology.
Qualified exams - GATE 2019,2020.
CAT EXAM 2021- 91.4 percentile
SSC EXAMS- 2019,2020,2021
AFCAT EXAM- 2019,2020,2021
I want to share my knowledge with other people so that they can achieve the same.
I have strong hold Mathematics, Electrical engineering and all the subjects related.
Just give me a problem and I will give you the solution of it.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A 0.446-g sample of an unknown monoprotic acid is titrated with 0.105 M KOH. The resulting titration curve is shown here. Determine the molar mass and pKa of the acid. Hd 14 12- 10- 06+NO 8- 4- 2 20...
-
A 0.1276-g sample of an unknown monoprotic acid was dissolved in 25.0 mL of water and titrated with 0.0633 M NaOH solution. The volume of base required to bring the solution to the equivalence point...
-
The following question is designed to highlight key concepts from the Loyalty Programs topic article titled, "StarBUCKS, Loyalty, and Breakage" (Nevraumont 2019). Q. Author's position. "If you hire...
-
Peters Company produces golf discs which it normally sells to retailers for $7 each. The cost of manufacturing 20,000 golf discs is: Materials ........ $ 10,000 Labor ......... 30,000 Variable...
-
Annys Dry Cleaners is owned and operated by Anny Brum. A building and equipment are currently being rented, pending expansion to new facilities. The actual work of dry cleaning is done by another...
-
What happens if you specify an invalid format string?
-
Ming Chen began a professional practice on June 1 and plans to prepare financial statements at the end of each month. During June, Ming Chen (the owner) completed these transactions: a. Owner...
-
Market Dynamics (10 Points) Between 1980 and 1990, the number of employed women grew sharply but the number of women employed as secretaries actually fell. There are two theories as to why this...
-
A 20.0-mL sample of 0.115 M sulfurous acid (H 2 SO 3 ) solution is titrated with 0.1014 M KOH. At what added volume of base solution does each equivalence point occur?
-
A 25.0-mL sample of 0.125 M pyridine is titrated with 0.100 M HCl. Calculate the pH at each volume of added acid: 0 mL, 10 mL, 20 mL, equivalence point, one-half equivalence point, 40 mL, 50 mL....
-
Suppose (a) Solve the (time-independent) Schrdinger equation for this potential. by letting z x and y(z) (1/) (x) (the a is just so y (z) is normalized with respect to z when (x) is normalized...
-
In a curling match, a 6.0kg rock with the speed of 3.50 m/s collides with another motionless 6.0kg rock. A] What are the velocities of the rocks after the collision if it is totally inelastic? B] How...
-
(Annuity number of periods) You've just bought a new flat-screen TV for $4,000 and the store you bought it from offers to let you finance the entire purchase at an annual rate of 16 percent...
-
1. Describe the health and safety requirements of the area in which the preventative maintenance activity is to take place, and the responsibility these requirements place on the learner 2. Describe...
-
Explain the significance of memorandum of association and articles of association in the formation and functioning of a company?
-
How do the four frames of color-blind racism contribute to the perpetuation of societal structures, particularly regarding the reproduction of racial inequalities and the reinforcement of dominant...
-
Summarize the differences between tax financing and bond financing, and, thinking like an economist and not a politician, explain the circumstances wherein each means of financing is best suited.
-
If a process has a six-sigma capability, what is the process capability index? a. 1 b. 2 c. 6 d. 12
-
A point source S is a perpendicular distance R away from the center of a circular hole of radius a in an opaque screen. If the distance from S to the periphery of the hole is (R + ), show that...
-
Starting with Eq. (9.53) for the transmitted wave, compute the flux density, that is, Eq. (9.54). , = Egelo tt' Eoeiot 1 pPe-i (9.53) 14(t')? I; (1 + r4) 2r (9.54) cos 8
-
A form of the Jamin Interferometer is illustrated in Fig. P.9.52. How does it work? To what use might it be put? Figure P.9.52
-
What is the percentage difference between the conversion rate and their opponents' conversion rate
-
Which modality interests you? Why does it interest you? What kind of patient conditions and disease processes are referred to your imaging modality? What are the educational, training & licensure...
-
The school of thought that views trade as the interplay between power, security and technology as forces that promote national strength is called: a. Institutionalism. b. Constitutionalism. c....

Study smarter with the SolutionInn App


