A 0.446-g sample of an unknown monoprotic acid is titrated with 0.105 M KOH. The resulting titration
Question:
A 0.446-g sample of an unknown monoprotic acid is titrated with 0.105 M KOH. The resulting titration curve is shown here.
Determine the molar mass and pKa of the acid.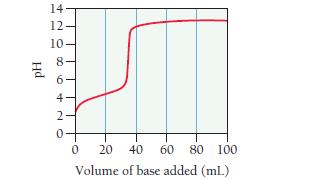
Transcribed Image Text:
Hd 14 12- 10- ∞06+NO 8- 4- 2 20 40 60 80 100 Volume of base added (mL) 0
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
To determine the molar mass of the acid 1 Calculate the moles of KOH used in the titration 2 Since t...View the full answer

Answered By

Shaira grace
I have experience of more than ten years in handing academic tasks and assisting students to handle academic challenges. My level of education and expertise allows me communicate eloquently with clients and therefore understanding their nature and solving it successfully.
5.00+
4+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A 0.229-g sample of an unknown monoprotic acid is titrated with 0.112 M NaOH. The resulting titration curve is shown here. Determine the molar mass and pK a of the acid. Hd 14 12- 10 8 6- Na 00 4- 0...
-
Find the analytic tan te=4+Pv. 37 co20-9 Coso +2.
-
A 0.5224-g sample of an unknown monoprotic acid was titrated with 0.0998 M NaOH. The equivalence point of the titration occurred at 23.82 mL. Determine the molar mass of the unknown acid.
-
Why " Kodak " is unsuccessful in implementing a strategy. Can you prepare a critical examination of the strategy to address the following questions about Kodak. What was the strategy and why do you...
-
Winters Inc. has been manufacturing its own shades for its table lamps. The company is currently operating at 100% of capacity. Variable manufacturing overhead is charged to production at the rate of...
-
A global equity manager is assigned to select stocks from a universe of large stocks throughout the world. The manager will be evaluated by comparing her returns to the return on the MSCI World...
-
What needs to be contained in the caption of a complaint?
-
Suppose that you are trying to decide between two job offers. One consulting firm offers you $150,000 per year to work out of its New York office. A second consulting firm wants you to work out of...
-
Topics: Analyzing and explicating for support or critique Assessment: Summarize expository and persuasive pieces Reading: Borgella, A. (2016). Science deconstructs humor: What makes some things...
-
A 20.0-mL sample of 0.115 M sulfurous acid (H 2 SO 3 ) solution is titrated with 0.1014 M KOH. At what added volume of base solution does each equivalence point occur?
-
A 25.0-mL sample of 0.125 M pyridine is titrated with 0.100 M HCl. Calculate the pH at each volume of added acid: 0 mL, 10 mL, 20 mL, equivalence point, one-half equivalence point, 40 mL, 50 mL....
-
Use the information provided in each row to calculate the missing values (shaded boxes). Each row is an independentsituation. Cost of sales inventory turnover ratio inventory on hand Average...
-
Mikaela's Consulting, a smaller business that advises other small businesses about marketing and online marketing, had net sales of $500,000 last year. After deducting cost of goods sold, operating...
-
Conduct research to find out one or more differences that exist between balance sheets that are prepared using U.S. GAAP and those prepared using International Financial Reporting Standards (IFRS)....
-
Suppose c = [1,2] in a maximization problem. Is d = [-2,1] an improving direction? How about d = [1,-1] and d [-1,1] = cd < 0 cd > 0 cd = 0
-
UNLV will be hosting an annual football camp for High School students. The camp will run for four weeks in the summer with each individual session lasting 1 week. The summer camp will be hosted by...
-
XYZ corporation has noticed that its competitors have their prices significantly lower. They have researched the process of the other competitors manufacturing processes, and they seem to be similar...
-
What is content analysis, and why is it useful to a forensic accountant?
-
If a process has a six-sigma capability, what is the process capability index? a. 1 b. 2 c. 6 d. 12
-
A glass camera lens with an index of 1.55 is to be coated with a cryolite film (n 1.30) to decrease the reflection of normally incident green light ( 0 = 500 nm). What thickness should be deposited...
-
Using Fig. 9.73, which depicts the geometry of the Shuttle radar interferometer, show that z(x) = h - r 1 cos θ Then use the Law of Cosines to establish that Eq. (9.108) is correct. Fig....
-
Given that the mirrors of a FabryPerot Interferometer have an amplitude reflection coefficient of r = 0.894 4, find (a) The coefficient of finesse, (b) The half-width, (c) The finesse, and, (d) The...
-
A box of apples of mass 22 kg slides 2.5 m down a ramp inclined 44 to the horizontal. The force of friction on the box has a magnitude of 79 N. The box starts from rest. What is the final speed of...
-
A rebuilt transmission in a crate sits on the floor; the total mass is 80 kg. The crate must be brought to the top of a loading dock by sliding it up a ramp 2.5 m long, inclined at 30% The shop...
-
A skier with a mass of 80 kg starts from rest at the top of a ski slope 75.0 m high. Assuming negligible friction between the skis and the snow, how fast is she going at the bottom of the slope? Now...

Study smarter with the SolutionInn App


