A common isotope used in medical imaging is technetium- 99m, which emits gamma rays. A sample initially
Question:
A common isotope used in medical imaging is technetium- 99m, which emits gamma rays.![]()
A sample initially containing 0.500 mg of technetium-99m is monitored as a function of time. Based on its rate of gamma ray emission, a graph, showing the mass of active technetium-99m as a function of time, is prepared. Study the graph and answer the questions that follow.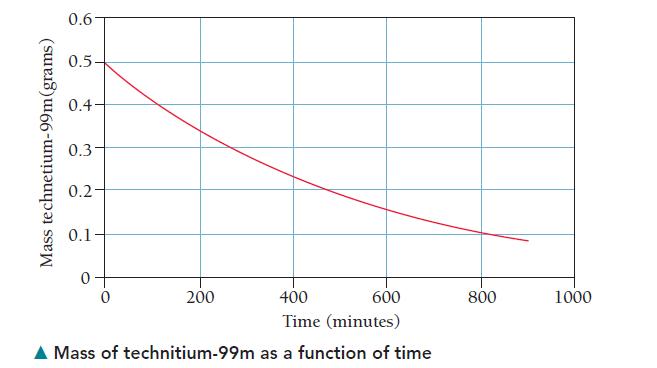
a. What is the mass of technetium-99m present at 200 minutes?
At 400 minutes?
b. What is the half-life of technetium-99m in minutes? In hours?
c. If a patient is given a 2.0-mg dose of technetium-99m, how much of it is left in the patient’s body after 10 hours? (For this problem, assume that the technetium-99m is not biologically removed from the body.)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





