A solution contains 3.95 g of carbon disulfide (CS 2 ) and 2.43 g of acetone (CH
Question:
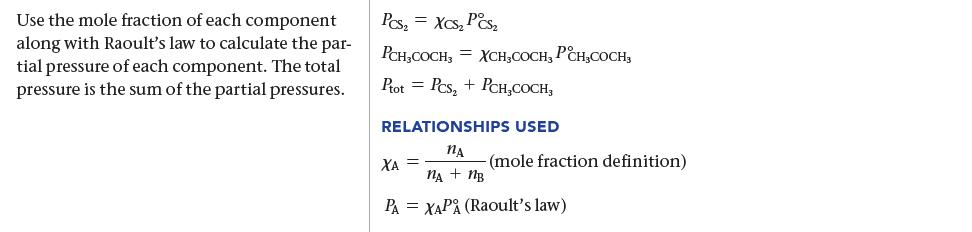
A solution contains 3.95 g of carbon disulfide (CS2) and 2.43 g of acetone (CH3COCH3). At 35 °C the vapor pressures of pure carbon disulfide and pure acetone are 515 torr and 332 torr, respectively. Assuming ideal behavior, calculate the vapor pressures of each component and the total vapor pressure above the solution. The experimentally measured total vapor pressure of the solution at 35 °C is 645 torr. Is the solution ideal? If not, what can you say about the relative strength of carbon disulfide–acetone interactions compared to the acetone–acetone and carbon disulfide–carbon disulfide interactions?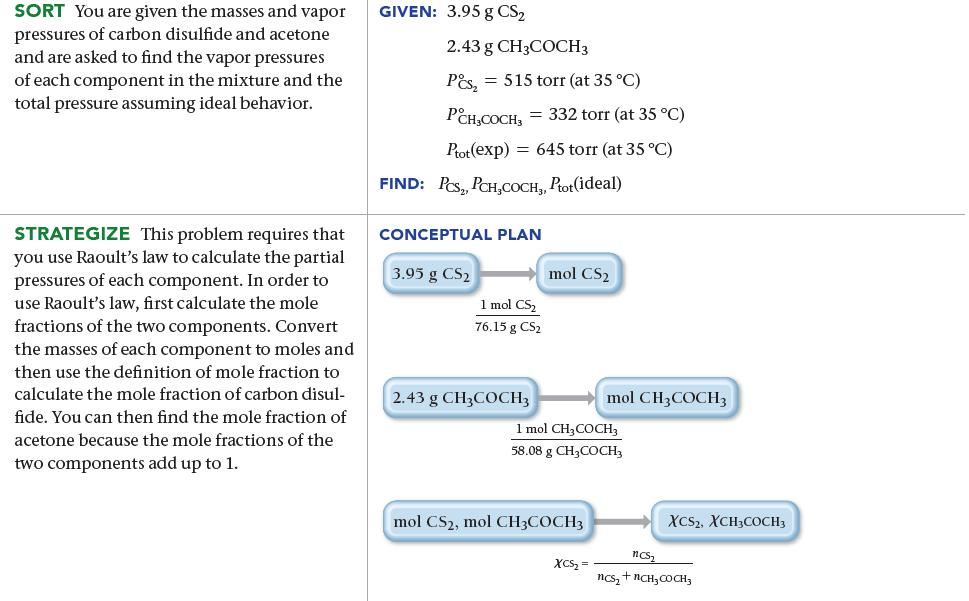
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





